476986
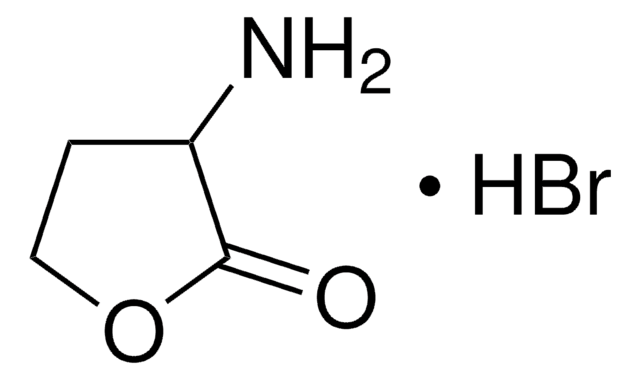
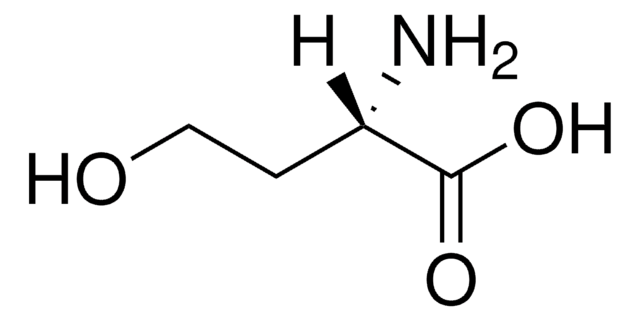
(S)-(+)-2-Amino-4-bromobutyric acid hydrobromide
97%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2CH2CH(NH2)CO2H · HBr
CAS Number:
Molecular Weight:
262.93
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
optical activity
[α]20/D +16°, c = 1 in methanol
mp
189 °C (dec.) (lit.)
functional group
amine
bromo
carboxylic acid
SMILES string
Br.N[C@@H](CCBr)C(O)=O
InChI
1S/C4H8BrNO2.BrH/c5-2-1-3(6)4(7)8;/h3H,1-2,6H2,(H,7,8);1H/t3-;/m0./s1
InChI key
JDLMXICGDYZOJH-DFWYDOINSA-N
Looking for similar products? Visit Product Comparison Guide
Application
(S)-(+)-2-Amino-4-bromobutyric acid hydrobromide may be used in the synthesis of metalloaminoacids such as [34S]-enriched methionine (34S-Met), telluromethionine (TeMet) and selenohomolanthionine (SeHLan).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Identification of selenohomolanthionine in selenium-enriched Japanese pungent radish.
Ogra Y, et al.
Journal of Analytical Atomic Spectrometry, 22(11), 1390-1396 (2007)
In vitro translation with [34S]-labeled methionine, selenomethionine, and telluromethionine.
Ogra Y, et al.
Analytical and Bioanalytical Chemistry, 390(1), 45-51 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service