429082
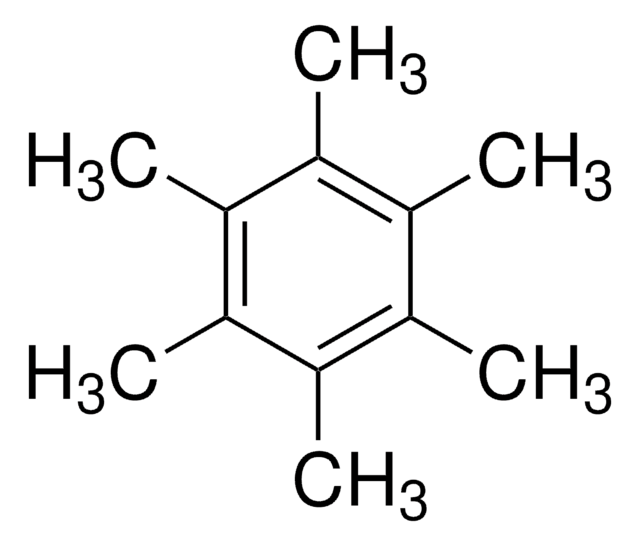
Hexamethylbenzene
purified by sublimation, ≥99%
Synonym(s):
1,2,3,4,5,6-Hexamethylbenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6(CH3)6
CAS Number:
Molecular Weight:
162.27
Beilstein/REAXYS Number:
1905834
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥99%
purified by
sublimation
bp
264 °C (lit.)
mp
164-166 °C (lit.)
SMILES string
Cc1c(C)c(C)c(C)c(C)c1C
InChI
1S/C12H18/c1-7-8(2)10(4)12(6)11(5)9(7)3/h1-6H3
InChI key
YUWFEBAXEOLKSG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Hexamethylbenzene (HMB) is a methyl benzene derivative. Its overtone spectra in carbon tetrachloride solution shows doublet structure due to two types of configurationally inequivalent hydrogens. The heat of solution of crystalline HMB has been obtained at 25°C from which the heat of fusion has been deduced by extrapolation process. The internal motion of the methyl groups has been explained by empirical force field calculations. Polarized Raman spectra of HMB at room temperature have been analyzed.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The Raman spectrum of hexamethylbenzene single crystal.
Girlando A and Pecile C.
Chemical Physics Letters, 20(5), 467-470 (1973)
On the gearing of methyl groups in hexamethylbenzene.
Iroff LD.
Journal of Computational Chemistry, 1(1), 76-80 (1980)
Detailed features in the local mode overtone bands of ethane, neopentane, tetramethylbutane, and hexamethylbenzene.
Henry BR and Greenlay WRA.
J. Chem. Phys., 72(10), 5516-5524 (1980)
The Heats of Solution of Hexamethylbenzene, Cetyl Alcohol, and Dicetyl in Related Liquids; Heats of Fusion by an Extrapolation Process.
Parks GS and Rowe RD.
J. Chem. Phys. , 14(9), 507-510 (1946)
Yaw Kai Yan et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 11(4), 483-488 (2006-04-11)
Ruthenium(II) arene anticancer complexes [(eta6-arene)Ru(en)Cl]PF6 (arene is hexamethylbenzene, p-cymene, indan; en is ethylenediamine) can catalyse regioselective reduction of NAD+ by formate in water to form 1,4-NADH, at pD 7.2, 37 degrees C, and in the presence of air. The catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service