427292
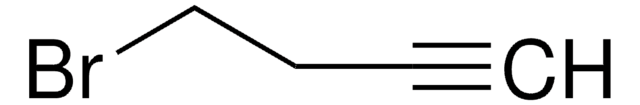
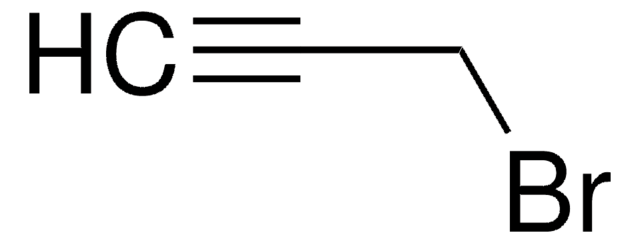
1-Bromo-2-butyne
≥98%
Synonym(s):
1-(Bromomethyl)-2-methylacetylene, 2-Butyn-1-yl bromide, 2-Butynyl bromide, 4-Bromobut-2-yne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
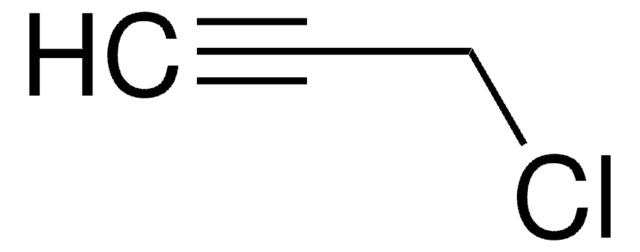
CH3C≡CCH2Br
CAS Number:
Molecular Weight:
132.99
Beilstein/REAXYS Number:
605306
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥98%
form
liquid
refractive index
n20/D 1.508 (lit.)
bp
40-41 °C/20 mmHg (lit.)
density
1.519 g/mL at 25 °C (lit.)
SMILES string
CC#CCBr
InChI
1S/C4H5Br/c1-2-3-4-5/h4H2,1H3
InChI key
LNNXOEHOXSYWLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Bromo-2-butyne is a propargyl bromide derivative. It is one of the constitutional isomer of bromo butyne. Its Br-loss threshold photoionization breakdown diagram has been analyzed to derive dissociative photoionization thresholds to C4H5+ production. It participates in the preparation of linagliptin.
Application
1-Bromo-2-butyne was used in the alkylation of L-tryptophan methyl ester. It was used as a source to generate CH3CCCH2 radicals to investigate the reaction kinetics of these radicals with NO and NO2.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
- 4-butynyloxybenzene sulfonyl chloride
- mono-propargylated diene derivative
- isopropylbut-2-ynylamine
- allenylcyclobutanol derivatives
- allyl-[4-(but-2-ynyloxy)phenyl]sulfane
- allenylindium
- alkynyl alcohols
- axially chiral teranyl compounds
Exploited in the synthesis of axially chiral teranyl compounds.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stereoselective synthesis of a-disubstituted cyclopentanones by palladium-catalyzed rearrangement of allenylcyclobutanols with aryl halides.
Yoshida M, et al.
Tetrahedron, 58(39), 7839-7846 (2002)
A chemical synthesis of 11-methoxy mitragynine pseudoindoxyl featuring the interrupted Ugi reaction.
Jimin Kim et al.
Chemical science, 3(9), 2849-2852 (2013-07-24)
A synthesis of 11-methoxy mitragynine pseudoindoxyl, a new member of the mitragynine class of opioid agonists, from a derivative of the Geissman-Waiss lactone is described. An internal attack of an electron-rich aromatic ring on an electrophilic nitrilium ion and a
Strained, Stable 2-Aza-1-Phosphabicyclo [n.1.0] alkane and-alkene Fe(CO)4 Complexes with Dynamic Phosphinidene Behavior.
Borst MLG, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 11(12), 3631-3642 (2005)
Exploring the functional space of thiiranes as gelatinase inhibitors using click chemistry.
Testero SA, et al.
ARKIVOC (Gainesville, FL, United States), 7, 221-236 (2011)
Reaction of zirconacyclopentadienes with electrophiles such as benzaldehyde, methyl methacrylate and 1-bromo-2-butyne after treatment with RLi.
Seki T, et al.
Tetrahedron Letters, 45(49), 9041-9043 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service