All Photos(2)
About This Item
Empirical Formula (Hill Notation):
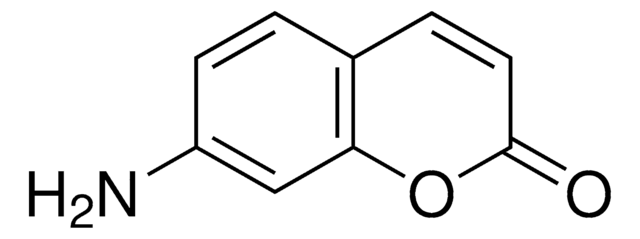
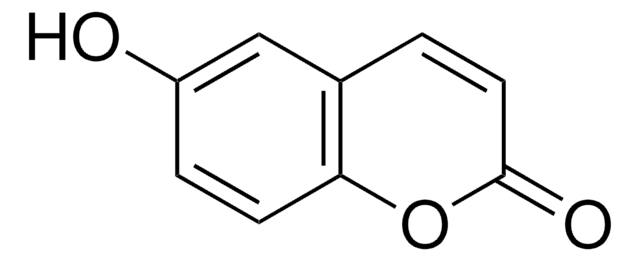
C9H7NO2
CAS Number:
Molecular Weight:
161.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
135-139 °C (lit.)
functional group
ester
SMILES string
NC1=Cc2ccccc2OC1=O
InChI
1S/C9H7NO2/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5H,10H2
InChI key
QWZHDKGQKYEBKK-UHFFFAOYSA-N
Application
3-Aminocoumarin may be employed in the following studies:
- As ligand for the synthesis of Cr(III), Ni(II), and Cu(II) complexes.
- As starting reagent for the synthesis of 1,2,3,4-tetrahydropyrido[2,3-c]coumarins.
- Synthesis of new 3-(heteroaryl)aminocoumarin derivatives, via optimized Buchwald-Hartwig amination reaction.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of biologically potent new 3-(heteroaryl) aminocoumarin derivatives via Buchwald-Hartwig C-N coupling.
Das AR, et al.
Tetrahedron Letters, 51(7), 1099-1102 (2010)
Fluorescence spectra of 3-aminocoumarin and its acid-base behaviour in the excited singlet state.
Rao RVS, et al.
Journal of Photochemistry, 34(1), 55-61 (1986)
Abdul Amir H Kadhum et al.
Molecules (Basel, Switzerland), 16(8), 6969-6984 (2011-08-17)
3-Aminocoumarin (L) has been synthesized and used as a ligand for the formation of Cr(III), Ni(II), and Cu(II) complexes. The chemical structures were characterized using different spectroscopic methods. The elemental analyses revealed that the complexes where M=Ni(II) and Cu(II) have
Iu A Vladimirov et al.
Biulleten' eksperimental'noi biologii i meditsiny, 112(10), 358-360 (1991-10-01)
The antioxidant capacity of 3-aminocoumarin, 3-oxycoumarin, 3-acetylaminocoumarin, and 3-coumarin carbonic acid has been investigated with chemiluminescence measurement and by the accumulation of TBA-active products. All coumarins were found to be antioxidants, with 3-oxy-, 3-amino- and 3-acetylamino coumarins being capable of
Hydrolysis-free synthesis of 3-aminocoumarins.
Kudale AA, et al.
Tetrahedron Letters, 48(29), 5077-5080 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service