384267
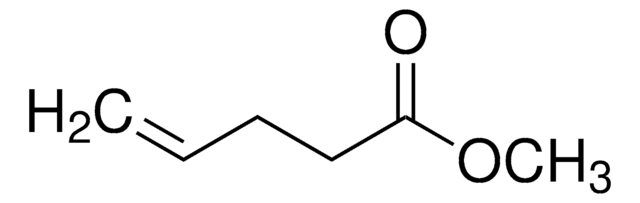
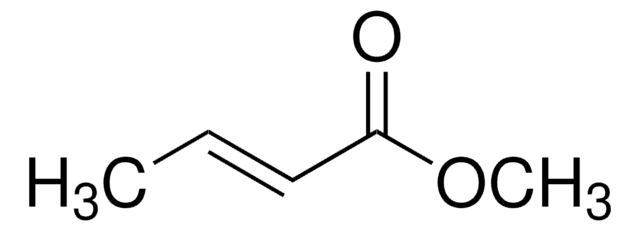
Methyl 3-butenoate
95%
Synonym(s):
Methyl vinylacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH2=CHCH2COOCH3
CAS Number:
Molecular Weight:
100.12
Beilstein/REAXYS Number:
1741732
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
assay:
95%
Recommended Products
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.409 (lit.)
bp
112 °C (lit.)
density
0.939 g/mL at 25 °C (lit.)
functional group
allyl
ester
SMILES string
COC(=O)CC=C
InChI
1S/C5H8O2/c1-3-4-5(6)7-2/h3H,1,4H2,2H3
InChI key
GITITJADGZYSRL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methyl 3-butenoate is an olefin ester. It is reported to undergo Iron carbonyl-promoted isomerization to afford α, β-unsaturated esters. It is one of the reaction products formed during flash vacuum thermolysis of (−)-cocaine. The H2 and CH4 chemical ionization mass spectra of methyl 3-butenoate has been reported.
Application
Methyl 3-butenoate may be employed for the synthesis of dipeptide olefin isosteres using intermolecular olefin cross-metathesis.
accessory
Product No.
Description
Pricing
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The flash vacuum thermolysis of (-)-cocaine.
Sisti NJ, et al.
Tetrahedron Letters, 30(44), 5977-5980 (1989)
Site of protonation in the chemical ionization mass spectra of olefinic methyl esters.
Harrison AX and Ichikawa H.
Org. Mass Spectrom., 15(5), 244-248 (1980)
Kuo-Chen Shih et al.
The Journal of organic chemistry, 61(22), 7784-7792 (1996-11-01)
Ultraviolet photolysis of stoichiometric amounts of methyl oleate and Fe(CO)(5) in hexanes solvent at 0 degrees C gives Fe(CO)(3)(eta(4)-alpha,beta-ester) in which the alpha,beta-unsaturated ester isomer of methyl oleate is stabilized by eta(4)-oxadiene pi coordination of the olefin and ester carbonyl
Melissa M Vasbinder et al.
The Journal of organic chemistry, 67(17), 6240-6242 (2002-08-17)
An approach to the synthesis of dipeptide olefin isosteres using intermolecular olefin cross-metathesis is presented. In particular, a synthesis of the Pro-Gly isostere (1) is reported. Conversion of N-BOC-proline into the corresponding vinyl-substituted carbamate provides the N-terminal cross-metathesis partner (2).
Rachel Chapla et al.
Polymers, 12(12) (2020-12-20)
Local mechanical stiffness influences cell behavior, and thus cell culture scaffolds should approximate the stiffness of the tissue type from which the cells are derived. In synthetic hydrogels, this has been difficult to achieve for very soft tissues such as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)