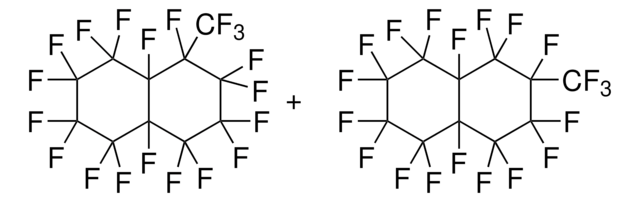
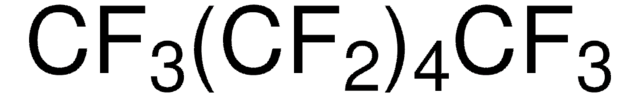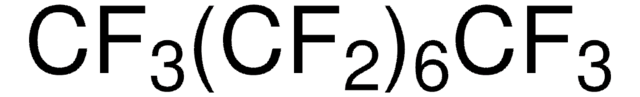379247
Tetradecafluorohexane
A mixture of perfluorinated hexanes, 95%
Synonym(s):
Perfluorohexane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CF3(CF2)4CF3
CAS Number:
Molecular Weight:
338.04
Beilstein/REAXYS Number:
1802113
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
liquid
impurities
5% perfluoropentane
refractive index
n20/D 1.252 (lit.)
bp
58-60 °C (lit.)
mp
-74 °C (lit.)
density
1.669 g/mL at 25 °C (lit.)
SMILES string
FC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F
InChI
1S/C6F14/c7-1(8,3(11,12)5(15,16)17)2(9,10)4(13,14)6(18,19)20
InChI key
ZJIJAJXFLBMLCK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Tetradecafluorohexane in the gas phase reacts spontaneously with lithium amalgam, to give a solid and intimate mixture of lithium fluoride and elemental polymeric carbon with a small amount of superstoichiometric lithium.
Application
Tetradecafluorohexane may be used as a fluorocarbon organic solvent in the preparation of temperature-induced phase-separation solution.
Other Notes
Contains perfluorocyclohexane
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 3
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Koichi Kitaguchi et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 30(6), 687-690 (2014-06-13)
A fluorocarbon and hydrocarbon organic solvent mixture is known as a temperature-induced phase-separation solution. When a mixed solution of tetradecafluorohexane as a fluorocarbon organic solvent and hexane as a hydrocarbon organic solvent (e.g., 71:29 volume ratio) was delivered in a
Yuko Kono et al.
Journal of vascular and interventional radiology : JVIR, 18(1 Pt 1), 57-65 (2007-02-14)
To determine whether contrast-enhanced ultrasound (CEUS) can aid in assessing treatment efficacy within the first 2 weeks after transarterial chemoembolization for hepatocellular carcinoma. Contrast-enhanced ultrasound was performed to detect residual tumor blood flow after 42 transarterial chemoembolization procedures in 33
Hermann Fromme et al.
Environmental science & technology, 41(22), 7928-7933 (2007-12-14)
Because dietary intake is supposed to be an important route of human exposure we quantified the dietary intake of perfluorooctane sulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexane sulfonate (PFHxS), perfluorohexanoate (PFHxA), and perfluorooctane sulfonamide (PFOSA) using 214 duplicate diet samples. The study
Ondine S von Ehrenstein et al.
Reproductive toxicology (Elmsford, N.Y.), 27(3-4), 239-245 (2009-05-12)
Polyfluoroalkyl chemicals (PFCs) comprise a group of man-made organic compounds, some of which are persistent contaminants with developmental toxicity shown in laboratory animals. There is a paucity of human perinatal exposure data. The US EPA conducted a pilot study (Methods
Kyunghee Ji et al.
Environment international, 45, 78-85 (2012-05-15)
Perfluorinated compounds (PFCs) have been frequently detected in both the environment and biota, and have become a growing concern. However, information is limited on the potential sources and human health implications of such exposure. We evaluated the exposure levels of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service