All Photos(2)
About This Item
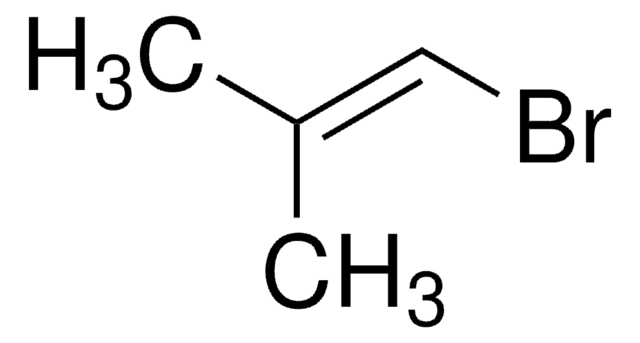
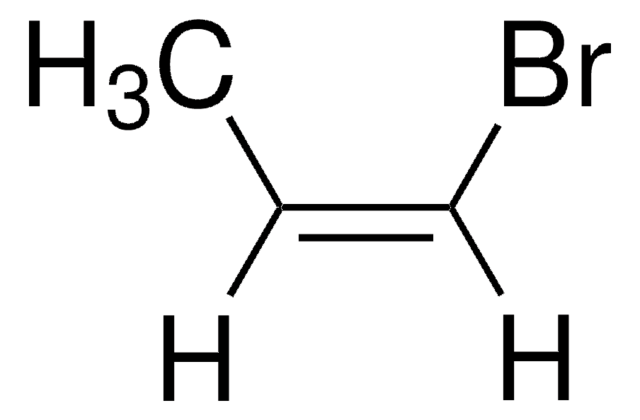
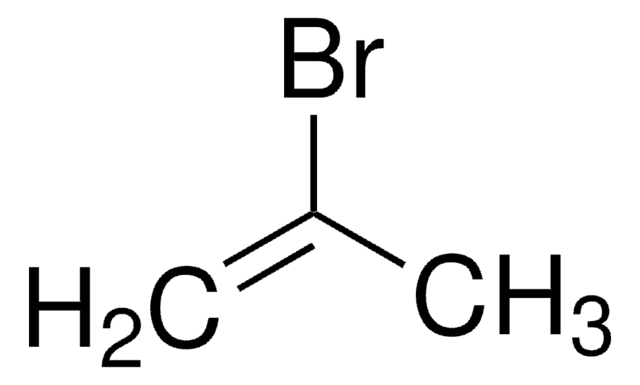
Linear Formula:
(CH3)2C=C(Br)CH3
CAS Number:
Molecular Weight:
149.03
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
96%
form
liquid
contains
copper pellets as stabilizer
refractive index
n20/D 1.474 (lit.)
bp
40 °C/75 mmHg (lit.)
density
1.284 g/mL at 25 °C (lit.)
functional group
alkyl halide
bromo
SMILES string
C\C(C)=C(\C)Br
InChI
1S/C5H9Br/c1-4(2)5(3)6/h1-3H3
InChI key
DBELOSOZLGEZBM-UHFFFAOYSA-N
General description
2-Bromo-3-methyl-2-butene is a vinylic bromide compound. Palladium/di-1-adamantyl-n-butylphosphine-catalyzed reductive carbonylation of 2-bromo-3-methyl-2-butene has been reported. Cross-coupling reaction of 2-bromo-3-methyl-2-butene with potassium 6-(benzoyloxy)hexyltrifluoroborate and 3-(benzoyloxy)propyltrifluoroborate has been investigated.
Application
2-Bromo-3-methyl-2-butene may be used in the preparation of:
- 2,3,4,5-tetramethyl-2,4-hexadiene
- 2-iodo-3-methyl-2-butene
- diastereomers of 2-amino-3-hydroxy-4,5-dimethylhexanoic acid
- lithium reagent, 2-lithio-3-methylbut-2-ene
- D-allo-(2R,3R,4R)-2-amino-3-hydroxy-4,5-dimethylhexanoic acid-containing peptide, pipecolidepsin A.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
77.0 °F
flash_point_c
25 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of All the Diastereomers of 2-Amino-3-hydroxy-4, 5-dimethylhexanoic Acid.
Spengler J and Albericio F.
European Journal of Organic Chemistry, 1, 44-47 (2014)
Synthesis and Molecular Structure of 2, 3, 4, 5-Tetramethyl-2, 4-hexadiene.
Br M, et al.
Acta Chemica Scandinavica. Series B, 31, 387-390 (1977)
Marta Pelay-Gimeno et al.
Nature communications, 4, 2352-2352 (2013-08-31)
Pipecolidepsin A is a head-to-side-chain cyclodepsipeptide isolated from the marine sponge Homophymia lamellosa. This compound shows relevant cytotoxic activity in three human tumour cell lines and has unique structural features, with an abundance of non-proteinogenic residues, including several intriguing amino
Palladium/di-1-adamantyl-n-butylphosphine-catalyzed reductive carbonylation of aryl and vinyl halides.
Brennfuhrer A, et al.
Tetrahedron, 63(27), 6252-6258 (2007)
Artis Klapars et al.
Journal of the American Chemical Society, 124(50), 14844-14845 (2002-12-12)
A mild and general method for the conversion of aryl, heteroaryl, and vinyl bromides into the corresponding iodides was developed utilizing a catalyst system comprising 5 mol % of CuI and 10 mol % of a 1,2- or 1,3-diamine ligand.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service