All Photos(2)
About This Item
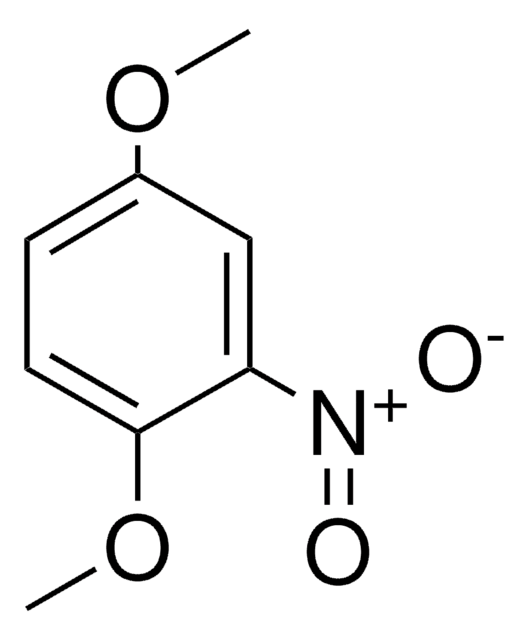
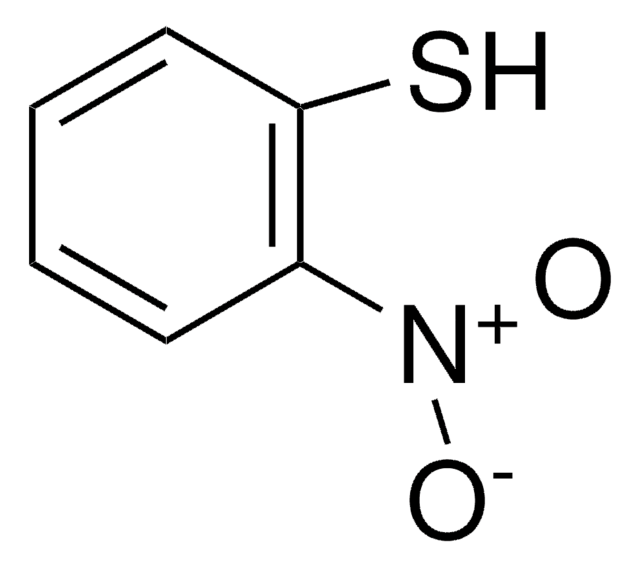
Linear Formula:
CH3C6H3(NO2)OCH3
CAS Number:
Molecular Weight:
167.16
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.557 (lit.)
bp
154 °C/14 mmHg (lit.)
mp
8-9 °C (lit.)
density
1.205 g/mL at 25 °C (lit.)
functional group
nitro
SMILES string
COc1ccc(C)cc1[N+]([O-])=O
InChI
1S/C8H9NO3/c1-6-3-4-8(12-2)7(5-6)9(10)11/h3-5H,1-2H3
InChI key
LGNMURXRPLMVJI-UHFFFAOYSA-N
Related Categories
General description
Nucleophilic substitution reactions of 4-methyl-2-nitroanisole in neat cyclohexylamine and piperidine have been reported.
Application
4-Methyl-2-nitroanisole may be used in the synthesis of 1-dibromomethyl-4-methoxy-2-nitrobenzene.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Theoretical calculations of chemical interactions. Part 4. Aromatic nucleophilic substitutions and SN 2 reactions of 4-and 6-substituted 2-nitroanisoles.
Nudelman NS and Palleros DR.
J. Chem. Soc. Perkin Trans. II, 6, 805-809 (1985)
Hoong-Kun Fun et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 9), o2193-o2194 (2009-01-01)
The asymmetric unit of the title compound, C(8)H(7)Br(2)NO(3), comprises two crystallographically independent mol-ecules (A and B). The nitro groups are twisted from the attached benzene rings, making dihedral angles of 39.26 (9) and 35.90 (9)° in mol-ecules A and B, respectively. In
Yusuke Yamamoto et al.
The Journal of toxicological sciences, 44(9), 585-600 (2019-09-03)
Amino acid derivative reactivity assay (ADRA) has previously been developed as an alternative method to direct peptide reactivity assay (DPRA) to evaluate key event 1 in skin sensitization mechanisms. However, when using alternative methods for skin sensitization, integrated approaches to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service