366765
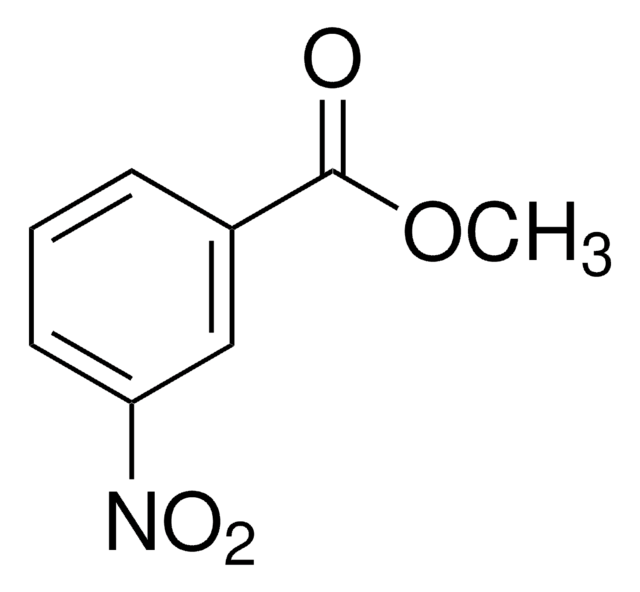
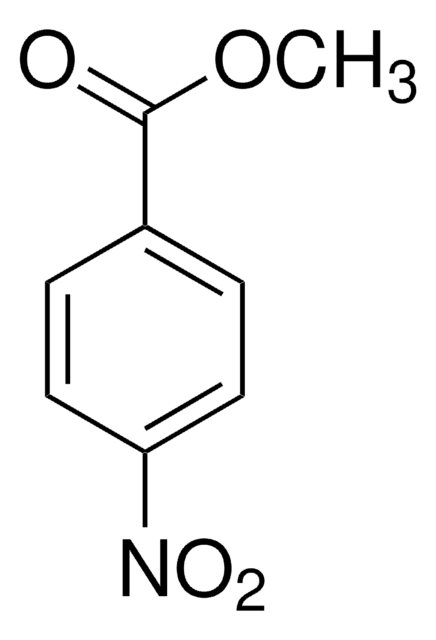
Methyl 2-methyl-3-nitrobenzoate
97%
Synonym(s):
Methyl 3-nitro-o-toluate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C6H3(NO2)CO2CH3
CAS Number:
Molecular Weight:
195.17
Beilstein/REAXYS Number:
1964020
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
solid
mp
62-65 °C (lit.)
functional group
ester
nitro
SMILES string
COC(=O)c1cccc(c1C)[N+]([O-])=O
InChI
1S/C9H9NO4/c1-6-7(9(11)14-2)4-3-5-8(6)10(12)13/h3-5H,1-2H3
InChI key
CRZGFIMLHZTLGT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Methyl 2-methyl-3-nitrobenzoate may be used in the synthesis of:
- methyl indole-4-carboxylate
- 5-aminoisoquinolin-1(2H)-one
- 5-nitroisocoumarin
- substituted nitrostyrene benzoic acids, via reaction with aromatic aldehydes in the presence of DBU in DMSO
- 4-(hydroxymethyl)-1-tosylindole, via Batcho-Leimgruber modification of the Reissert indole synthesis
- [2-(4-fluorophenyl)-1H-indol-4-yl]-1-pyrrolidinylmethanone
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 2-Arylindole-4-Carboxylic Amides:[2-(4-Fluorophenyl)-1H-Indol-4-YL]-1-Pyrrolidinylmethanone.
Kuethe JT and Beutner GL.
Organic Syntheses, 92-104 (2009)
A N Brubaker et al.
Journal of medicinal chemistry, 29(8), 1528-1531 (1986-08-01)
Three 7-methyl-1,4-dioxa-7-azaspiro[4.5]decanes that contained either the benzyl, 3-indolylmethyl, or 4-indolylmethyl group at the 6-position were synthesized via alkylation of the pyrrolidine enamine of the key intermediate, ethyl 3-oxopiperidine-1-carboxylate. The spirodecane derivatives were evaluated for in vivo central and peripheral dopamine
The Chemistry of Indoles. XII. A Facile Route to 5-Nitroisocoumarins and Methyl Indole-4-carboxylate.
<BIG>Masanori S, et al. </BIG>
Chemical & Pharmaceutical Bulletin, 29(1), 249-253 (1981)
Preparation of 2-arylindole-4-carboxylic amide derivatives.
Kuethe JT and Davies LW.
Tetrahedron, 62(49), 11381-11390 (2006)
M C McDonald et al.
British journal of pharmacology, 130(4), 843-850 (2000-06-24)
Poly (ADP-ribose) synthetase (PARP) is a nuclear enzyme activated by strand breaks in DNA, which are caused inter alia by reactive oxygen species (ROS). Here we report on (i) a new synthesis of a water-soluble and potent PARP inhibitor, 5-aminoisoquinolinone
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service