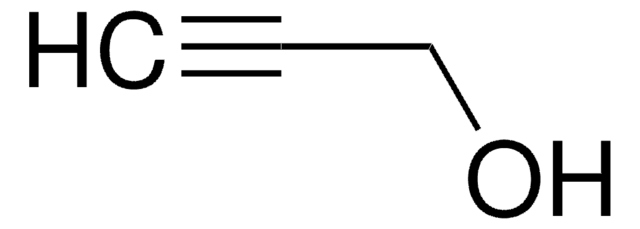363901
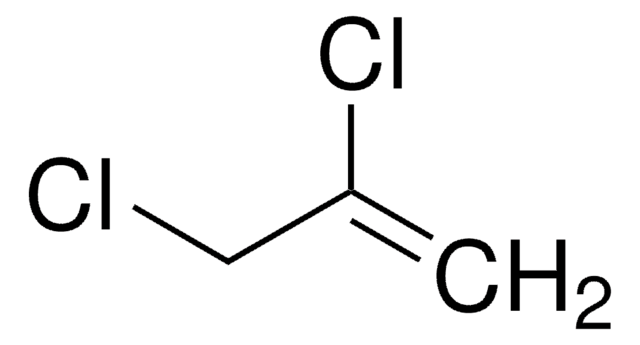
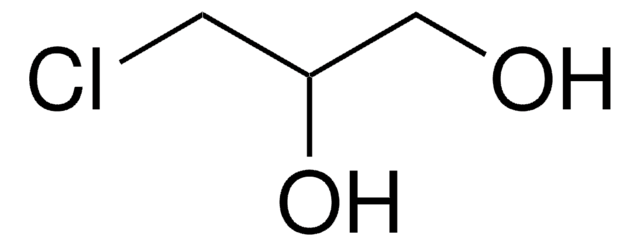
2-Chloro-2-propen-1-ol
technical grade, 90%
Synonym(s):
2-Chloroallyl alcohol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=C(Cl)CH2OH
CAS Number:
Molecular Weight:
92.52
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
assay
90%
form
liquid
refractive index
n20/D 1.459 (lit.)
bp
133-134 °C (lit.)
density
1.162 g/mL at 25 °C (lit.)
functional group
chloro
hydroxyl
SMILES string
OCC(Cl)=C
InChI
1S/C3H5ClO/c1-3(4)2-5/h5H,1-2H2
InChI key
OSCXYTRISGREIM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Chloro-2-propen-1-ol is reported to undergo photodissociation at 193nm to generate CH2CCH2OH radical intermediate. 2-Chloro-2-propen-1-ol is formed as major product during base mediated reaction of 1,2,3-trichloropropane. 2-Chloro-2-propen-1-ol is reported to react with phosphorus trichloride to yield phosphorous esters, while with phosphory chloride it yields phosphoric ester.
Application
2-Chloro-2-propen-1-ol (2-chloropropenol) may be employed as carbon supplement for the growth of Pseudomonas strains. It may be used in the preparation of 2-(4-octylphenyl)prop-2-en-1-ol.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
129.2 °F - closed cup
flash_point_c
54 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetic studies of the homogeneous abiotic reactions of several chlorinated aliphatic compounds in aqueous solution.
Pagan M, et al.
Applied Geochemistry, 13(6), 779-785 (1998)
J J van der Waarde et al.
Applied and environmental microbiology, 59(2), 528-535 (1993-02-01)
Three Pseudomonas strains capable of utilizing 2-chloroallylalcohol (2-chloropropenol) as the sole carbon source for growth were isolated from soil. The fastest growth was observed with strain JD2, with a generation time of 3.6 h. Degradation of 2-chloroallylalcohol was accompanied by
Ran Zhu et al.
Journal of medicinal chemistry, 50(25), 6428-6435 (2007-11-13)
Compound 1 (FTY720, Fingolimod) represents a new generation of immunosuppressant that modulates lymphocyte trafficking by interacting with the S1P(1) receptor. Compound 1 also provides a template molecule for studying the molecular biology of S1P receptors and related enzymes (kinases and
Derivatives of phosphorus acids and 2-chloro-2-propen-1-ol.
Arbuzov BA, et al.
Russian Chemical Bulletin, 16(6), 1233-1238 (1967)
Arjun S Raman et al.
The Journal of chemical physics, 127(15), 154316-154316 (2007-10-24)
These velocity map imaging experiments characterize the photolytic generation of one of the two radical intermediates formed when OH reacts via an addition mechanism with allene. The CH2CCH2OH radical intermediate is generated photolytically from the photodissociation of 2-chloro-2-propen-1-ol at 193
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service