All Photos(3)
About This Item
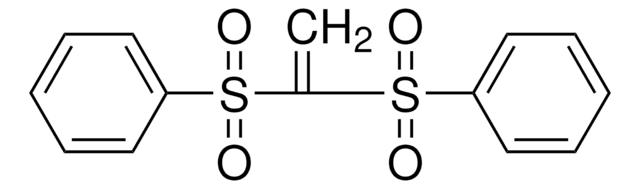
Linear Formula:
C6H5SO2CH=CHSO2C6H5
CAS Number:
Molecular Weight:
308.37
Beilstein/REAXYS Number:
2334889
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
mp
221-223 °C (lit.)
functional group
sulfone
SMILES string
O=S(=O)(\C=C\S(=O)(=O)c1ccccc1)c2ccccc2
InChI
1S/C14H12O4S2/c15-19(16,13-7-3-1-4-8-13)11-12-20(17,18)14-9-5-2-6-10-14/h1-12H/b12-11+
InChI key
YGBXMKGCEHIWMO-VAWYXSNFSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Chiral BINAP-AuTFA- and chiral BINAP-AgTFA (TFA = trifluoroacetate anion) -promoted catalytic enantioselective 1,3-dipolar cycloadditions of iminoglycinates with trans-1,2-bis(phenylsulfonyl)ethylene were studied.
Application
trans-1,2-Bis(phenylsulfonyl)ethylene was used in total synthesis of (+)-7-deoxypancratistatin.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Binap-gold (I) trifluoroacetate as a bifunctional catalyst for the synthesis of chiral prolines through 1, 3-dipolar cycloaddition of azomethine ylides.
Martin-Rodriguez M, et al.
Tetrahedron Asymmetry, 21(9), 1184-1186 (2010)
J L Aceña et al.
Organic letters, 2(23), 3683-3686 (2000-11-14)
A new total synthesis of (+)-7-deoxypancratistatin 1 has been accomplished in 19 steps (8% overall yield) from two readly available compounds, furan and trans-1,2-bis(phenylsulfonyl)ethylene.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service