32950
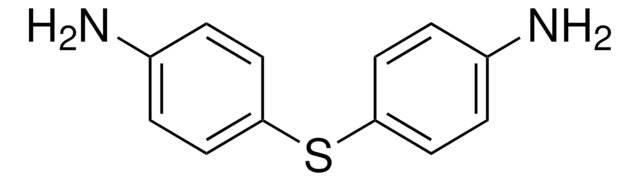
4,4′-Diaminodiphenylmethane
≥97.0% (GC)
Synonym(s):
4,4′-Methylenedianiline, MDA
About This Item
Recommended Products
Quality Level
assay
≥97.0% (GC)
mp
88-92 °C
solubility
water: soluble
SMILES string
Nc1ccc(Cc2ccc(N)cc2)cc1
InChI
1S/C13H14N2/c14-12-5-1-10(2-6-12)9-11-3-7-13(15)8-4-11/h1-8H,9,14-15H2
InChI key
YBRVSVVVWCFQMG-UHFFFAOYSA-N
Gene Information
mouse ... Slc6a2(20538)
rat ... Slc6a3(24898) , Slc6a4(25553)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as dummy template for bisphenols imprinting
- in the synthesis of amino modified multi-walled carbon nanotubes/polydimethylsiloxane
- as curing agent to study the kinetics of formation of epoxy resin composites
- in rubber industry as an epoxyresin hardener
signalword
Danger
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 1B - Muta. 2 - Skin Sens. 1 - STOT RE 2 - STOT SE 1
target_organs
Liver, Liver,eye - retina
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
429.8 °F - closed cup
flash_point_c
221 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service