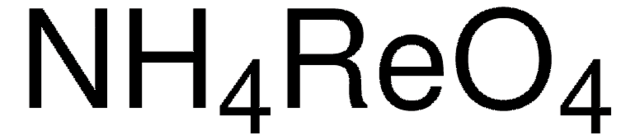316954
Ammonium perrhenate
≥99%
Synonym(s):
Metaperrhenic acid ammonium salt, Rhenium standard solution
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
NH4ReO4
CAS Number:
Molecular Weight:
268.24
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
assay
≥99%
form
powder or crystals
reaction suitability
reagent type: catalyst
core: rhenium
density
3.97 g/mL at 25 °C (lit.)
SMILES string
N.O[Re](=O)(=O)=O
InChI
1S/H3N.H2O.3O.Re/h1H3;1H2;;;;/q;;;;;+1/p-1
InChI key
XPSXHXWHJAIQJR-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Ammonium perrhenate is a white crystalline solid soluble in ethanol and water. It is a weak oxidizer and widely used as a Re precursor in nanomaterial synthesis and Re-based catalyst preparation.
Application
Ammonium perrhenate used:
- As a rhenium source to prepare Re nanoparticles for catalytic decomposition of ammonium perchlorate.
- To synthesize ReS2 through a direct sulfidation reaction, for the fabrication of field effect transistors (FETs), digital logic devices, humidity sensors, and rechargeable supercapacitors.
- As a precursor to prepare the heterogeneous catalysts for organic transformations.
- An excellent precursor for the fabrication of ReOx/TiO2 recyclable solidcatalyst for deoxydehydration. For at least six runs in a row, the catalyst demonstrated catalyticactivity, selectivity, and stability without deactivation.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Perez-Lourido, P. et al.
Inorganic Chemistry, 38, 1511-1511 (1999)
Jeffrey G Linger et al.
Proceedings of the National Academy of Sciences of the United States of America, 111(33), 12013-12018 (2014-08-06)
Lignin is an energy-dense, heterogeneous polymer comprised of phenylpropanoid monomers used by plants for structure, water transport, and defense, and it is the second most abundant biopolymer on Earth after cellulose. In production of fuels and chemicals from biomass, lignin
Rui Cao et al.
Inorganic chemistry, 50(19), 9499-9507 (2011-08-30)
We describe a multidentate tripodal ligand in which three pendant arms carrying di(2-picolyl)amine units are linked to the ortho positions of a tris(o-xylyl) scaffold, providing N(CH(2)-o-C(6)H(4)CH(2)N(CH(2)py)(2))(3) (L). Reaction of L with CuCl(2) in the presence of hexafluorophosphate anion afforded blue
Olesya A Nikonova et al.
Langmuir : the ACS journal of surfaces and colloids, 27(18), 11622-11628 (2011-08-13)
The use of an inorganic perrhenate ligand in the structure of early-transition-metal alkoxide precursors permits to achieve uniform self-assembly of the primary nanoparticles produced by their hydrolysis. The latter has been carried out in a hydrocarbon reaction medium by the
David P Cormode et al.
Dalton transactions (Cambridge, England : 2003), 39(28), 6532-6541 (2010-06-17)
A disulfide functionalized bis-ferrocene urea acyclic receptor and disulfide functionalized mono- and bis-ferrocene amide and urea appended upper rim calix[4]arene receptors were prepared for the fabrication of SAM redox-active anion sensors. 1H NMR and diffusive voltammetric anion recognition investigations revealed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service