292206
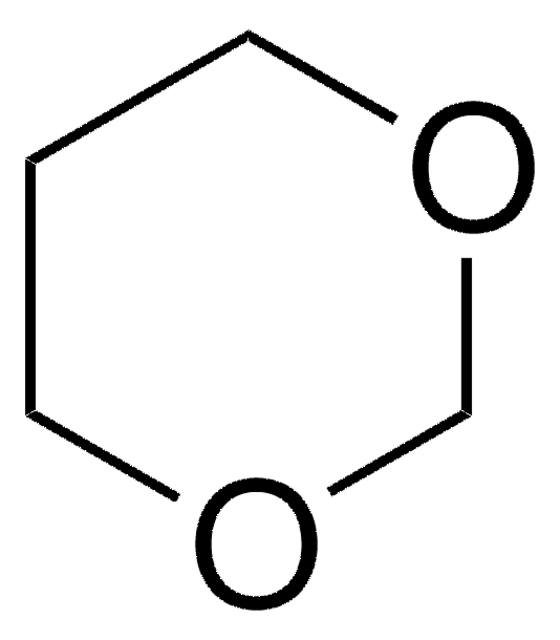
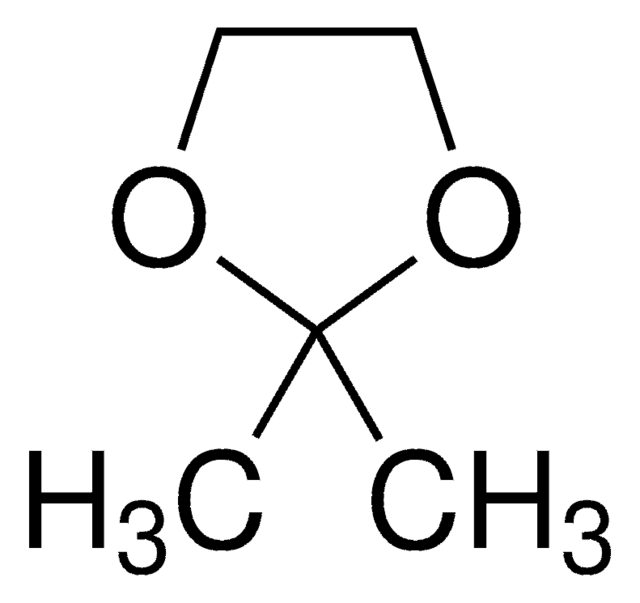
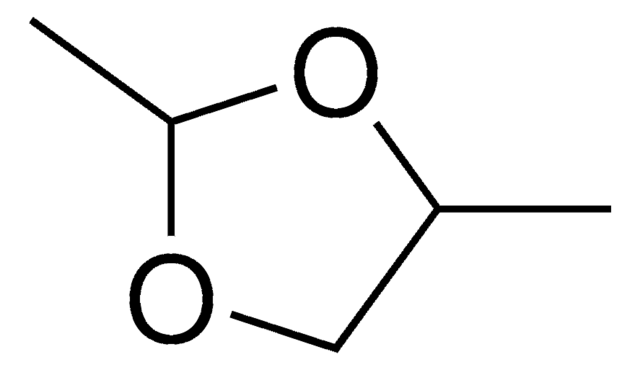
2-Methyl-1,3-dioxolane
97%
Synonym(s):
Acetaldehyde ethylene acetal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H8O2
CAS Number:
Molecular Weight:
88.11
Beilstein/REAXYS Number:
102520
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.398 (lit.)
bp
82-83 °C (lit.)
density
0.982 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CC1OCCO1
InChI
1S/C4H8O2/c1-4-5-2-3-6-4/h4H,2-3H2,1H3
InChI key
HTWIZMNMTWYQRN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The kinetics and mechanism of the gas-phase thermal decomposition of 2-methyl-1,3-dioxolane has been studied in a static system. The infrared spectra of solid, liquid and gaseous 2-methyl-1,3-dioxolane has been studied. Low-temperature ozonation of 2-methyl-1,3-dioxolane in acetone-d6, methyl acetate and tert-butyl methyl ether has been reported.
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
28.4 °F - closed cup
flash_point_c
-2 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T R Ward et al.
Toxicology and applied pharmacology, 122(2), 300-307 (1993-10-01)
Some compounds that inhibit acetylcholinesterase (AChE) activity compete directly with quinuclidinyl benzilate (QNB) binding, a muscarinic antagonist which binds to all subtypes equally, and with cis-methyldioxolane (CD), an agonist that binds with high affinity to the M2 subtype of muscarinic
S Yamada et al.
Brain research, 410(2), 212-218 (1987-05-05)
To study the role of central cholinergic mechanisms in hypertension, we have determined nicotinic and muscarinic agonist binding sites in the brain regions of stroke-prone spontaneously hypertensive rats (SHRSP), using [3H]nicotine and [3H]cismethyldioxolane (CD). There was a significant decrease in
H Tecle et al.
Life sciences, 52(5-6), 505-511 (1993-01-01)
The synthesis of a series of potent and efficacious 1-azabicyclo[2.2.1]heptan-3-one oxime muscarinic agonists is described. The oximes have extended appendages designed to span the cavity defined by the seven transmembrane helices of the muscarinic receptor. Some members of the series
T W Vickroy et al.
Federation proceedings, 43(13), 2785-2790 (1984-10-01)
The binding of agonists to muscarinic cholinergic receptors is well described by a binding model of multiple affinity states (superhigh, high, and low) in most central and peripheral tissues. Although previous studies of the influences by divalent cations, guanine nucleotides
T R Ward et al.
Brain research bulletin, 39(1), 49-55 (1996-01-01)
Recent reports indicate that organophosphate insecticides, in addition to inhibiting acetylcholinesterase activity, can bind directly at a subset of muscarinic receptors, which also bind cis-methyldioxolane with high affinity. Muscarinic receptors are known to act through at least two second messenger
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service