287288
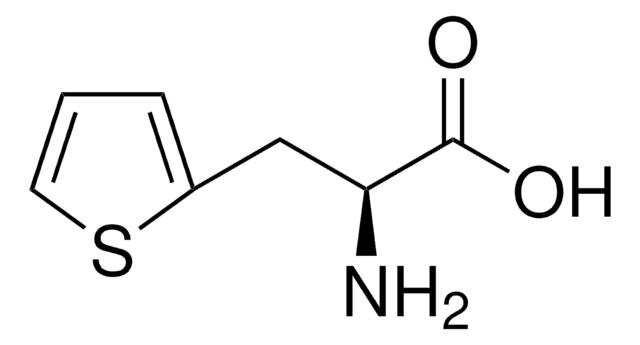
3-(2-Thienyl)-DL-alanine
≥98%
Synonym(s):
2-Amino-3-(2-thienyl)propionic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H9NO2S
CAS Number:
Molecular Weight:
171.22
Beilstein/REAXYS Number:
82874
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
≥98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
275-277 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NC(Cc1cccs1)C(O)=O
InChI
1S/C7H9NO2S/c8-6(7(9)10)4-5-2-1-3-11-5/h1-3,6H,4,8H2,(H,9,10)
InChI key
WTOFYLAWDLQMBZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K J Brown et al.
Medical microbiology and immunology, 168(1), 11-24 (1980-02-01)
Faecal samples were taken from three diet-managed phenylketonuric children to determine effects of beta-2-thienylalanine (beta-2-t) on indigenous bacteria. From sample swabs, 127 anaerobes were identified and tested for beta-2-t inhibition on a phenylalanine (Phe)-free medium, Anaerobe Inhibition Test (AIT) agar.
I W Hamley et al.
The journal of physical chemistry. B, 114(32), 10674-10683 (2010-07-29)
The self-assembly of a peptide based on a sequence from the amyloid beta peptide but incorporating the non-natural amino acid beta-2-thienylalanine (2-Thi) has been investigated in aqueous and methanol solutions. The peptide AAKLVFF was used as a design motif, replacing
D G Adams et al.
Critical reviews in microbiology, 9(1), 45-100 (1981-01-01)
We will be concerned with the two major differentiated cell types of filamentous cyanobacteria--the heterocyst and the akinete. The former is generally accepted to be the site of aerobic nitrogen fixation in heterocystous cyanobacteria. The latter is a spore-like cell
Uptake and incorporation of amino acids by suspension cultured mammalian cells: a comparative study involving eleven naturally-occurring and four analogue amino acids.
D N Wheatley et al.
Cytobios, 30(118), 101-126 (1981-01-01)
D P Bedard et al.
Journal of bacteriology, 141(1), 100-105 (1980-01-01)
When treated with the amino acid analog beta-2-DL-thienylalanine, cells of the yeast Saccharomyces cerevisiae accumulated in the G1 portion of the cell cycle at the "start" event. This G1 arrest was accompanied by a rapid decrease in the rate of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service