All Photos(1)
About This Item
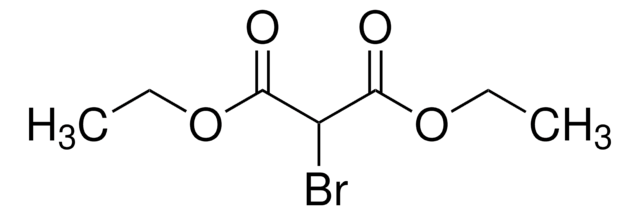
Linear Formula:
FCH(COOC2H5)2
CAS Number:
Molecular Weight:
178.16
Beilstein/REAXYS Number:
1775686
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
form
liquid
refractive index
n20/D 1.407 (lit.)
bp
121-122 °C/30 mmHg (lit.)
density
1.129 g/mL at 25 °C (lit.)
functional group
ester
fluoro
SMILES string
CCOC(=O)C(F)C(=O)OCC
InChI
1S/C7H11FO4/c1-3-11-6(9)5(8)7(10)12-4-2/h5H,3-4H2,1-2H3
InChI key
GOWQBFVDZPZZFA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The reaction of diethyl fluoromalonate with electron-poor and electron-rich, sterically hindered and unhindered aryl bromides and chlorides were studied.
Application
The kinetic parameters of diethyl fluoromalonate in hemolysates were used to study carboxylesterase activities in complex systems.
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
143.6 °F - closed cup
flash_point_c
62 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G L Mendz et al.
Archives of biochemistry and biophysics, 305(2), 252-260 (1993-09-01)
Eight fluorinated compounds were tested as putative probes to measure carboxylesterase activity employing 19F nuclear magnetic resonance spectroscopy. The method takes advantage of the sensitivity of fluorine resonances to the changes in the chemical bonding in the covalent structures where
Neil A Beare et al.
The Journal of organic chemistry, 67(2), 541-555 (2002-01-19)
Palladium-catalyzed reactions of aryl bromides and chlorides with two common stabilized carbanions-enolates of dialkyl malonates and alkyl cyanoesters-are reported. An exploration of the scope of these reactions was conducted, and the processes were shown to occur in a general fashion.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service