276030
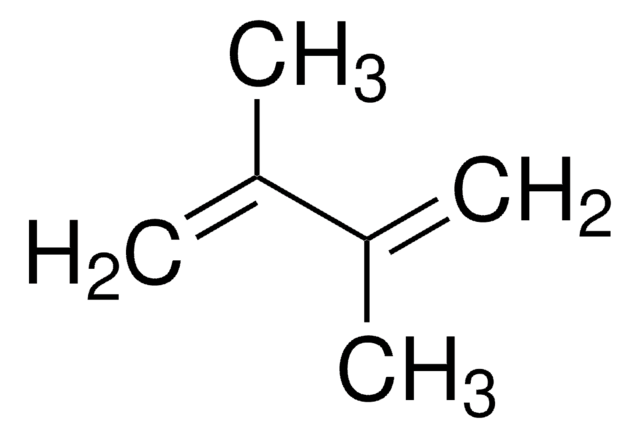
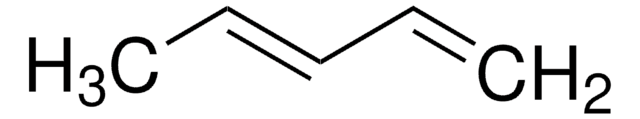
2,3-Dimethoxy-1,3-butadiene
95%
Synonym(s):
2,3-Dimethoxybuta-1,3-diene, 2,3-Dimethoxybutadiene
About This Item
Recommended Products
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.459 (lit.)
bp
134-136 °C/745 mmHg (lit.)
mp
19 °C (lit.)
density
0.94 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
COC(=C)C(=C)OC
InChI
1S/C6H10O2/c1-5(7-3)6(2)8-4/h1-2H2,3-4H3
InChI key
NHBDKDZHQKQPTF-UHFFFAOYSA-N
General description
Application
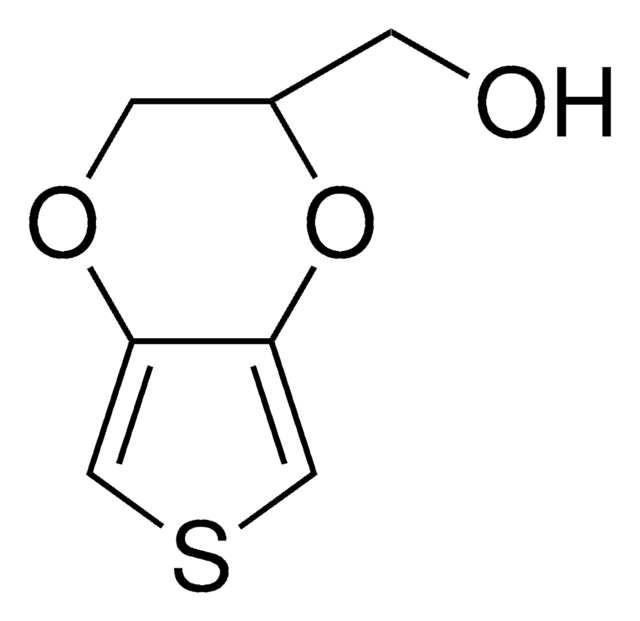
It may be used in the preparation of 3,4-dimethoxythiophene, an intermediate for the synthesis of 3,4-ethylenedioxythiophene (EDOT). It may also be used to form thio esters by reacting with mercaptans in the presence of cobalt carbonyl catalyst.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. Since the reaction involves the formation of a cyclic product via a cyclic transition state, it is also referred to as a "cycloaddition".
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service