262579
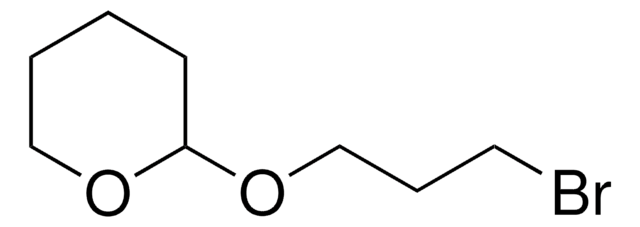
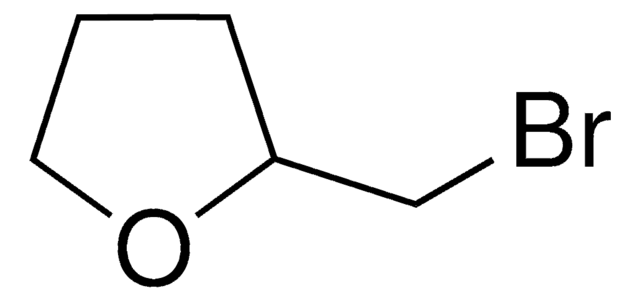
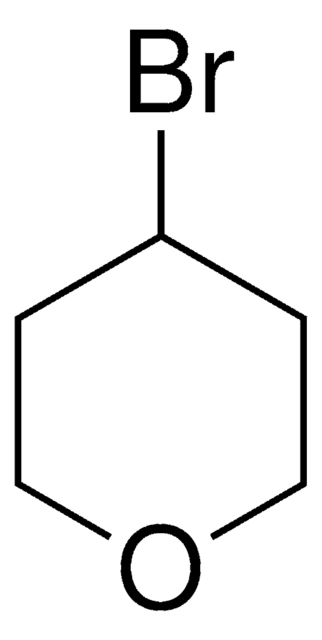
2-(Bromomethyl)tetrahydro-2H-pyran
98%
Synonym(s):
Tetrahydro-2H-pyran-2-methyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11BrO
CAS Number:
Molecular Weight:
179.05
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.489 (lit.)
bp
153 °C (lit.)
mp
−59-−58 °C (lit.)
density
1.397 g/mL at 25 °C (lit.)
functional group
bromo
ether
SMILES string
BrCC1CCCCO1
InChI
1S/C6H11BrO/c7-5-6-3-1-2-4-8-6/h6H,1-5H2
InChI key
MHNWCBOXPOLLIB-UHFFFAOYSA-N
General description
Cross-coupling reaction of 2-(bromomethyl)tetrahydro-2H-pyran with potassium heteroaryltrifluoroborates has been investigated.
Application
2-(Bromomethyl)tetrahydro-2H-pyran has been used in the preparation of tellurated heterocycles, 2-[(2-thienyltelluro)methyl]tetrahydrofuran and [(2-thienyltelluro)methyl]tetrahydro-2H-pyran.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
149.0 °F - closed cup
flash_point_c
65 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tellurated heterocycles, 2-[(2-thienyltelluro)methyl]tetrahydrofuran and [(2-thienyltelluro)methyl]tetrahydro-2H-pyran: Synthesis and complexation reactions with Pd(II), Pt(II), Hg(II), Ru(II) and Cu(I)
Bali S, et al.
Journal of Organometallic Chemistry, 691(18), 3788-3796 (2006)
Gary A Molander et al.
Organic letters, 12(24), 5783-5785 (2010-11-26)
A method for the cross-coupling of alkyl electrophiles with various potassium aryl- and heteroaryltrifluoroborates has been developed. Nearly stoichiometric amounts of organoboron species could be employed to cross-couple a large variety of challenging heteroaryl nucleophiles. Several functional groups were tolerated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service