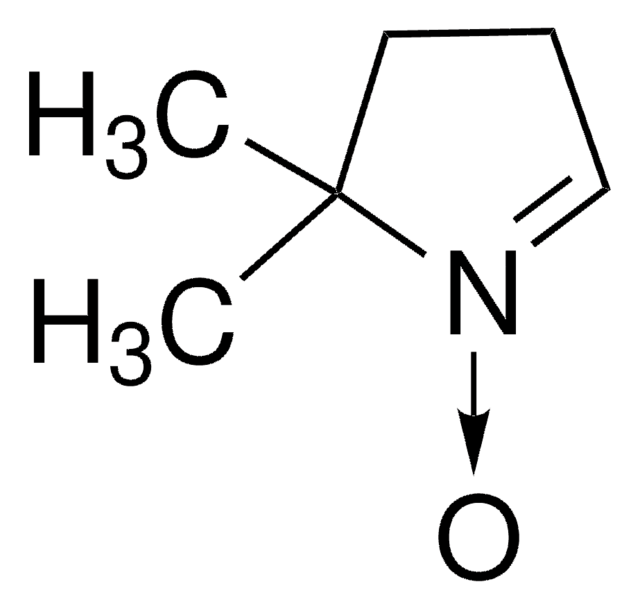
214000
TEMPO
98%
Synonym(s):
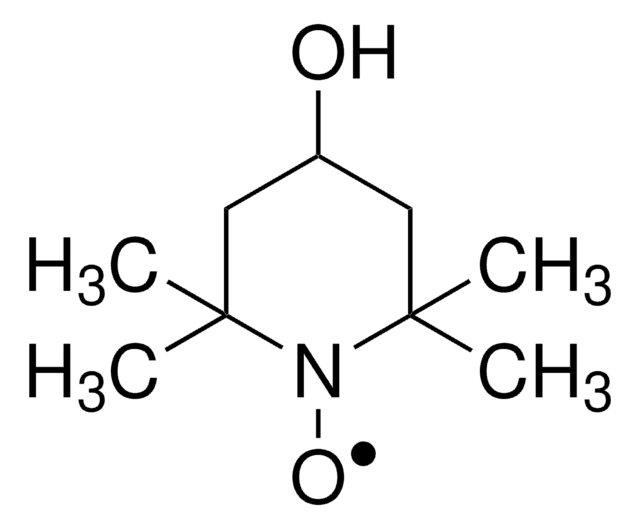
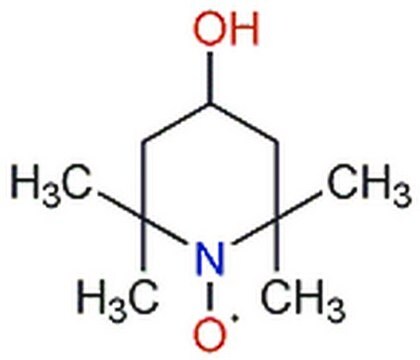
2,2,6,6-Tetramethylpiperidine 1-oxyl, 2,2,6,6-Tetramethyl-1-piperidinyloxy, free radical, TEMPO
About This Item
Recommended Products
Quality Level
assay
98%
form
solid
reaction suitability
reagent type: oxidant
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
36-38 °C (lit.)
greener alternative category
storage temp.
2-8°C
SMILES string
CC1(C)CCCC(C)(C)N1[O]
InChI
1S/C9H18NO/c1-8(2)6-5-7-9(3,4)10(8)11/h5-7H2,1-4H3
InChI key
QYTDEUPAUMOIOP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a catalytic oxidant for copper-catalyzed, greener oxidation of alcohols under aerobic conditions.
- As a catalytic oxidant in the iodobenzene diacetae oxidation of nerol to neral.
- For trapping the styrenyl radical generated from benzoyl peroxide during nitroxide-mediated radical polymerization of styrene.
- In the carboxylation of water-resistant nanofibrillated cellulose (NFC) films.
Copper(I)/TEMPO-catalyzed aerobic oxidation of primary alcohols to aldehydes with ambient air
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1C
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 2
flash_point_f
152.6 °F - closed cup
flash_point_c
67 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Related Content
he Stahl Lab focuses on the development of catalysts and catalytic reactions for selective oxidation of organic molecules, with particular emphasis on aerobic oxidation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service