199176
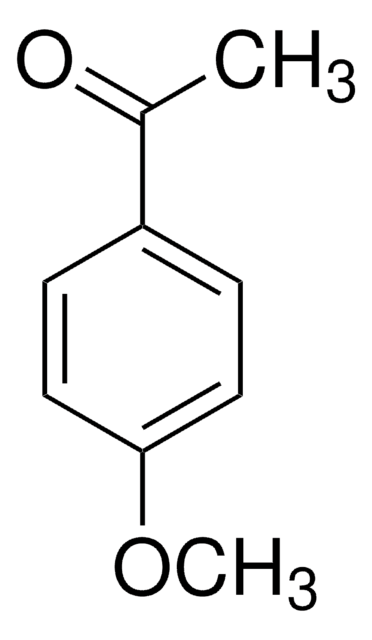
4-Methoxyphenylacetone
≥97%
Synonym(s):
4-Methoxybenzyl methyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CH2COCH3
CAS Number:
Molecular Weight:
164.20
Beilstein/REAXYS Number:
2044332
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
≥97%
form
liquid
refractive index
n20/D 1.525 (lit.)
bp
145 °C/25 mmHg (lit.)
density
1.067 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
COc1ccc(CC(C)=O)cc1
InChI
1S/C10H12O2/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6H,7H2,1-2H3
InChI key
WFWKNGZODAOLEO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Asymmetric amination of 4-methoxyphenylacetone was studied.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
215.6 °F - closed cup
flash_point_c
102 °C - closed cup
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Asymmetric amination of 4-methoxyphenylacetone and its related compounds with microorganisms.
Applied Microbiology and Biotechnology, 33(6), 637-640 (1990)
Hamid Sadeghian et al.
Bioorganic & medicinal chemistry, 17(6), 2327-2335 (2009-03-03)
A group of 4-methoxyphenylacetic acid esters were designed, synthesized and evaluated as potential inhibitors of soybean 15-lipoxygenase (SLO) on the basis of eugenol and esteragol structures. Compounds 7d-e showed the best IC(50) in SLO inhibition (IC(50)=3.8 and 1.9 microM, respectively).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service