179701
Triethylborane solution
1.0 M in THF
Synonym(s):
Triethylboron
About This Item
Recommended Products
form
liquid
reaction suitability
reagent type: reductant
concentration
1.0 M in THF
density
0.865 g/mL at 25 °C
SMILES string
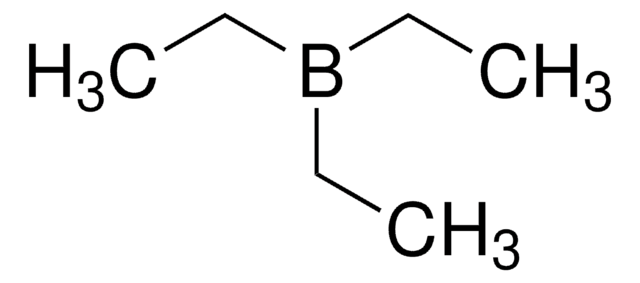
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
LALRXNPLTWZJIJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
It can be employed as a reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes and for the synthesis of tetramethylammonium trialkylphenylborate salts.
signalword
Danger
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1A - STOT SE 3 - Water-react 2
target_organs
Central nervous system, Respiratory system
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 1
flash_point_f
1.4 °F - closed cup
flash_point_c
-17 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service