160644
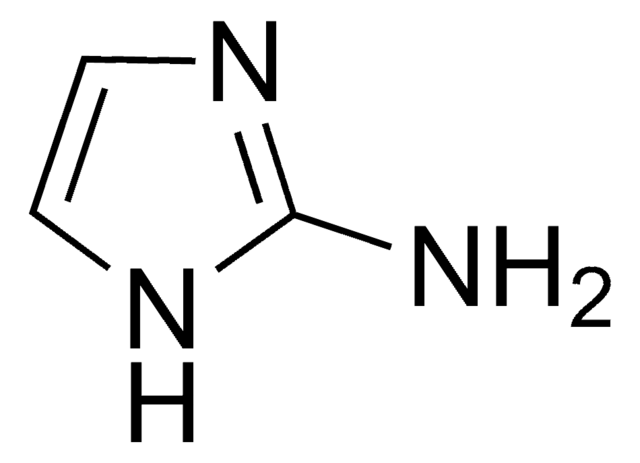
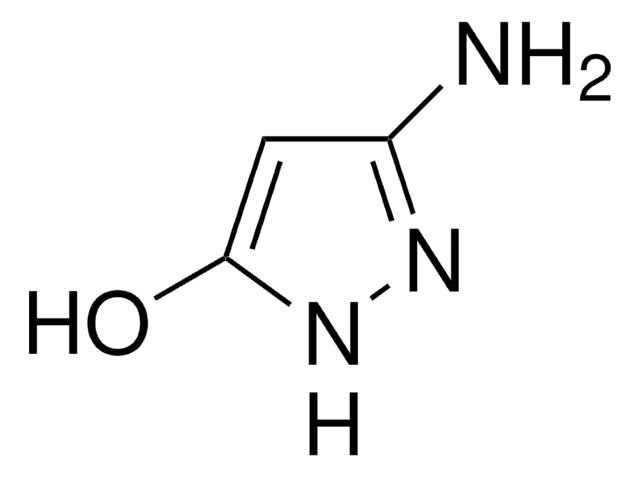
3-Aminopyrazole
98%
Synonym(s):
3-AP, 3-Pyrazolamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H5N3
CAS Number:
Molecular Weight:
83.09
Beilstein/REAXYS Number:
1618
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
solid
bp
218 °C/122 mmHg (lit.)
mp
34-37 °C (lit.)
SMILES string
Nc1cc[nH]n1
InChI
1S/C3H5N3/c4-3-1-2-5-6-3/h1-2H,(H3,4,5,6)
InChI key
JVVRJMXHNUAPHW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Aminopyrazole is a heteroarylamine.
Application
3-Aminopyrazole was used in the spectroscopic characterization of ferrocenoyl peptides via 1H-NMR spectroscopy. It was also used in the synthesis of:
- symmetrical dialkylpyrazolo[1,5-a]pyrimidines via condensation with symmetrical β-diketones
- 3,4-annelated coumarins
- heterocyclic compounds of pharmaceutical interest
- pyrazolopyrimidines
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T Novinson et al.
Journal of medicinal chemistry, 18(5), 460-464 (1975-05-01)
A number of 3-bromo-, 3-nitro-, and 3-ethoxycarbonyl-5,7-dialkylpyrazolo[1,5-a]pyrimidines were synthesized and screened as in vitro cAMP phosphodiesterase inhibitors. The condensation of 3-aminopyrazole with symmetrical beta-diketones (acetylacetone, heptane-3,5-dione, etc.) afforded symmetrical dialkylpyrazolo[1,5-a]pyrimidines (5). The reaction of 3-aminopyrazole with unsymmetrical beta-diketones (hexane-2,4-dione, heptane-3,5-dione
The interaction of ferrocenoyl peptides with 3-aminopyrazole.
Saweczko P and Kraatz H-B.
Coordination Chemistry Reviews, 190, 185-198 (1999)
Tetrahedron Letters, 47, 2611-2611 (2006)
Trends Heterocycl. Chem., 2, 97-97 (1991)
Synthesis and structure of some 3, 4-annelated coumarin systems.
Govori S, et al.
Heterocyclic Communications, 8(2), 129-134 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service