154385
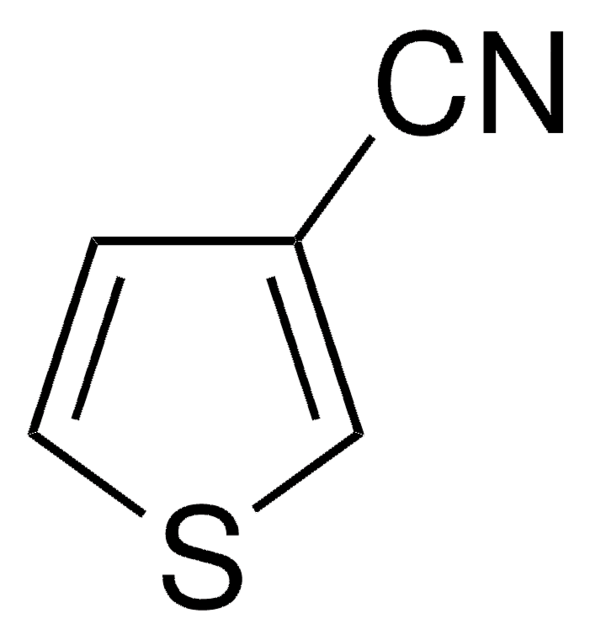
2-Thiophenecarbonitrile
99%
Synonym(s):
2-Cyanothiophene, Thiophene-2-carbonitrile
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H3NS
CAS Number:
Molecular Weight:
109.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
liquid
refractive index
n20/D 1.563 (lit.)
bp
192 °C (lit.)
density
1.172 g/mL at 25 °C (lit.)
functional group
nitrile
SMILES string
N#Cc1cccs1
InChI
1S/C5H3NS/c6-4-5-2-1-3-7-5/h1-3H
InChI key
CUPOOAWTRIURFT-UHFFFAOYSA-N
Application
2-Thiophenecarbonitrile (2-Cyanothiophene) was used in the preparation of thiaplatinacycles. It was also used in the synthesis of 2,2′-thienylpyrroles.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
127.4 °F - closed cup
flash_point_c
53 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ming Yu et al.
Organic letters, 6(6), 1057-1059 (2004-03-12)
[reaction: see text] Two new series of 2,2'-bipyrroles and 2,2'-thienylpyrroles have been prepared by trimethylsilyl trifluoromethanesulfonate (TMSOTf)-mediated reaction of donor-acceptor cyclopropanes with 2-cyanopyrroles and 2-cyanothiophene, respectively. This method opens the door toward a wide variety of unsymmetrical bipyrroles and thienylpyrroles.
Tülay A Ateşin et al.
Inorganic chemistry, 47(11), 4596-4604 (2008-05-02)
The reaction of 2-cyanothiophene with a zerovalent platinum bisalkylphosphine fragment yields two thiaplatinacycles derived from the cleavage of the substituted and unsubstituted C-S bonds. While cleavage away from the cyano group is preferred kinetically, cleavage adjacent to the cyano group
Muhammad Ajmal et al.
Journal of colloid and interface science, 470, 39-46 (2016-03-02)
In this study, the synthesis of micron-sized poly(vinylbenzyl chloride) (p(VBC)) beads and subsequent conversion of the reactive chloromethyl groups to double amidoxime group containing moieties by post modification is reported. The prepared beads were characterized by SEM and FT-IR spectroscopy.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service