All Photos(1)
About This Item
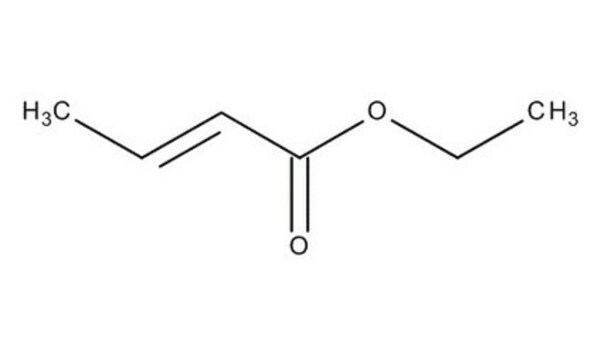
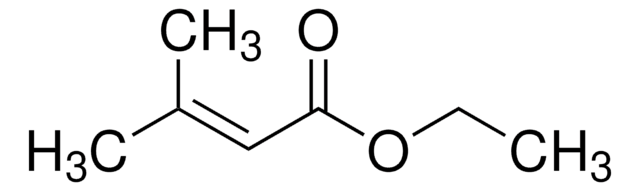
Linear Formula:
CH3CH=CHCOOCH3
CAS Number:
Molecular Weight:
100.12
Beilstein/REAXYS Number:
1720292
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
118-120 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
COC(=O)\C=C\C
InChI
1S/C5H8O2/c1-3-4-5(6)7-2/h3-4H,1-2H3/b4-3+
InChI key
MCVVUJPXSBQTRZ-ONEGZZNKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Methyl crotonate undergoes vinylogous aldol reaction with enolizable aldehydes in the presence of aluminum tris(2,6-di-2-naphthylphenoxide).
Application
Methyl crotonate was used to investigate chemoselectivity in the reaction between methyl crotonate and benzylamine catalyzed by lipase B from Candida antarctica using solvent engineering. It was used as starting reagent during the total synthesis of phytotoxins solanapyrones D(1) and E(2).
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 2
flash_point_f
30.2 °F - closed cup
flash_point_c
-1 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jeffrey A Gazaille et al.
Organic letters, 14(11), 2678-2681 (2012-05-25)
The synthesis of the novel Lewis acid, aluminum tris(2,6-di-2-naphthylphenoxide) (ATNP), and its use in the vinylogous aldol reaction between methyl crotonate and enolizable aldehydes are described. ATNP is related to Yamamoto's Lewis acid, aluminum tris(2,6-diphenylphenoxide) (ATPH), but the 2-naphthyl groups
Solvent engineering: an effective tool to direct chemoselectivity in a lipase-catalyzed Michael addition.
Priego J, et al.
Tetrahedron, 65(2), 536-539 (2009)
Hisahiro Hagiwara et al.
The Journal of organic chemistry, 67(17), 5969-5976 (2002-08-17)
The phytotoxins solanapyrones D (1) and E (2) have been synthesized from the decalone prepared by the domino Michael reaction of the kinetic enolate of optically pure acetylcyclohexene with methyl crotonate. The decalone was transformed into a solanapyrone core by
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 139459-500ML | 4061836682354 |
| 139459-100ML | 4061838732866 |
| 139459-5ML | 4061838732873 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service