139351
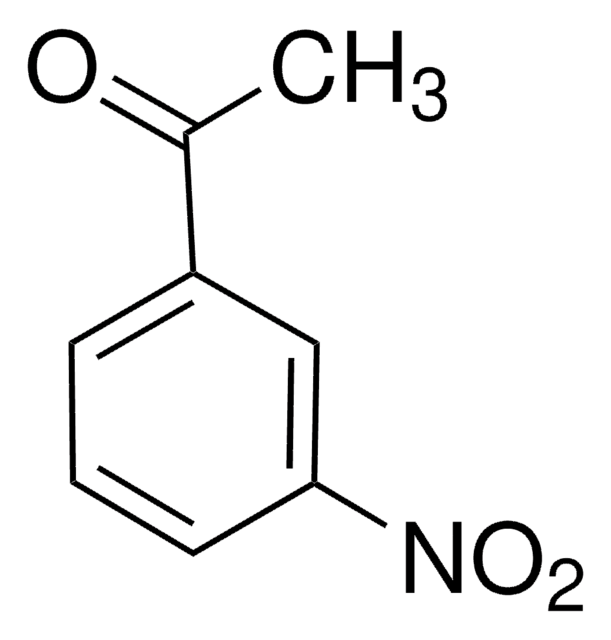
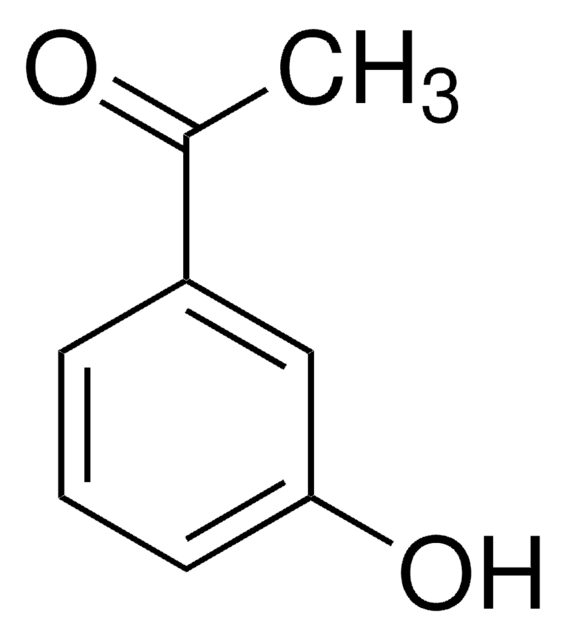
3′-Aminoacetophenone
97%
Synonym(s):
3-Acetylaniline
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H4COCH3
CAS Number:
Molecular Weight:
135.16
Beilstein/REAXYS Number:
386009
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
bp
289-290 °C (lit.)
mp
94-98 °C (lit.)
SMILES string
CC(=O)c1cccc(N)c1
InChI
1S/C8H9NO/c1-6(10)7-3-2-4-8(9)5-7/h2-5H,9H2,1H3
InChI key
CKQHAYFOPRIUOM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3′-Aminoacetophenone acts as bifunctional coupling reagent during the synthesis of pyrimidines.
Application
3′-Aminoacetophenone (3-Acetylaniline) was used as reagent during the asymmetric total synthesis of pactamycin. It was used as starting reagent during the synthesis of curcumin mimics with substituted sulfonyl group.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M K Subramanian et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 71(1), 59-67 (2008-01-08)
The gas phase infrared spectrum of 3-aminoacetophenone (3AAP) was measured in the range 5000-500 cm(-1) and with a resolution of 0.5 cm(-1). The Fourier transform Raman (FT-Raman) and Fourier transform infrared (FT-IR) spectra of 3AAP were recorded in the solid
Chan Mug Ahn et al.
Bioorganic & medicinal chemistry letters, 19(5), 1481-1483 (2009-01-31)
In order to discover novel small vasodilatory molecules for potential use in the treatment of vascular disease, we tested the vasodilatation effect of two types of synthetic curcumin mimics, amide type (3) and sulfonyl amide type (4), upon the basilar
J S Früchtel et al.
Biotechnology and bioengineering, 71(2), 94-103 (2001-04-05)
A combination of symmetric building blocks and combinatorial functional group transformation for synthesis of pyrimidines was investigated. The purpose of the study was to maximize the return on invested synthetic efforts of reaction development for libraries. A representative set of
Justin T Malinowski et al.
Science (New York, N.Y.), 340(6129), 180-182 (2013-04-13)
Medicinal application of many complex natural products is precluded by the impracticality of their chemical synthesis. Pactamycin, the most structurally intricate aminocyclopentitol antibiotic, displays potent antiproliferative properties across multiple phylogenetic domains, but it is highly cytotoxic. A limited number of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service