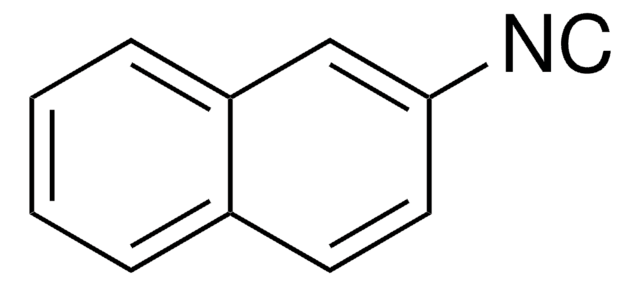
133299
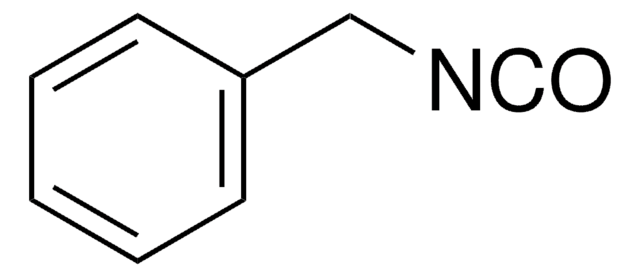
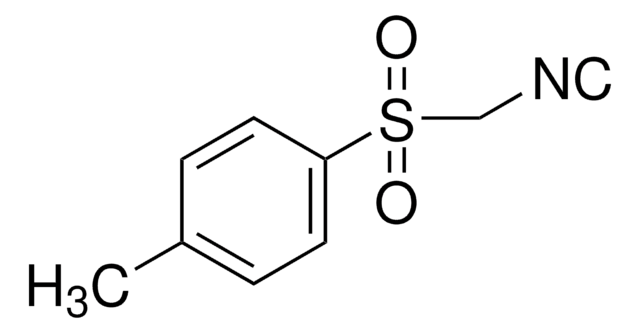
Benzyl isocyanide
98%
Synonym(s):
(Isocyanomethyl)benzene, Benzyl isonitrile
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2NC
CAS Number:
Molecular Weight:
117.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
refractive index
n20/D 1.521 (lit.)
bp
105-106 °C/75 mmHg (lit.)
density
0.962 g/mL at 25 °C (lit.)
functional group
amine
isonitrile
phenyl
storage temp.
−20°C
SMILES string
[C-]#[N+]Cc1ccccc1
InChI
1S/C8H7N/c1-9-7-8-5-3-2-4-6-8/h2-6H,7H2
InChI key
RIWNFZUWWRVGEU-UHFFFAOYSA-N
Related Categories
General description
Benzyl isocyanide forms phosphaalkene-containing complexes.
Application
Benzyl isocyanide was used in the synthesis of Ru(II) complexes containing hydrazine and benzyl isocyanide ligands. It was used in a three-component coupling process leading to O- and N-arylamides.
Other Notes
May darken in storage
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
174.2 °F - closed cup
flash_point_c
79 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Samantha N MacMillan et al.
Chemical communications (Cambridge, England), (40)(40), 4172-4174 (2007-10-11)
Reaction of (N(3)N)ZrPHPh (N(3)N=N(CH(2)CH(2)NSiMe(3))(3)(3-)) with PhCH(2)N[triple bond]C affords the 1,1-insertion product (N(3)N)Zr[C(PHPh)=NCH(2)Ph], which thermally rearranges to the phosphaalkene-containing complex, (N(3)N)Zr[N(CH(2)Ph)C(H)=PPh].
Laurent El Kaïm et al.
The Journal of organic chemistry, 72(11), 4169-4180 (2007-04-26)
The use of Smiles rearrangement in Ugi- and Passerini-type couplings with electron-deficient phenols allows very straightforward multicomponent formation of O-aryl- and N-arylamides. Best yields were observed with the highly activated o- and p-nitrophenols, salicylic derivatives giving adducts in lower yields.
Synthesis and X-ray studies of ruthenium (II) complexes containing hydrazine and benzyl isocyanide ligands.
Owalude SO, et al.
Bulletin of the Chemical Society of Ethiopia, 27(3), 405-411 (2013)
Zeinab Faghih et al.
Iranian journal of pharmaceutical research : IJPR, 19(3), 134-143 (2021-03-09)
The complex [(PhCH2NC)AuCl], 1, was prepared by the reaction of [(Me2S)AuCl], A, with an equimolar amount of benzyl isocyanide (PhCH2NC) ligand. Through a salt metathesis reaction, the chloride ligand in 1 was replaced by potassium benzothiazole-2-thiolate (Kbt) and potassium benzoimidazole-2-thiolate
Ben J Tickner et al.
Chemical science, 10(20), 5235-5245 (2019-06-14)
We report the formation of a series of novel [Ir(H)2(IMes)(α-13C2-carboxyimine)L] complexes in which the identity of the coligand L is varied. When examined with para-hydrogen, complexes in which L is benzylamine or phenethylamine show significant 1H hydride and 13C2 imine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service