All Photos(1)
About This Item
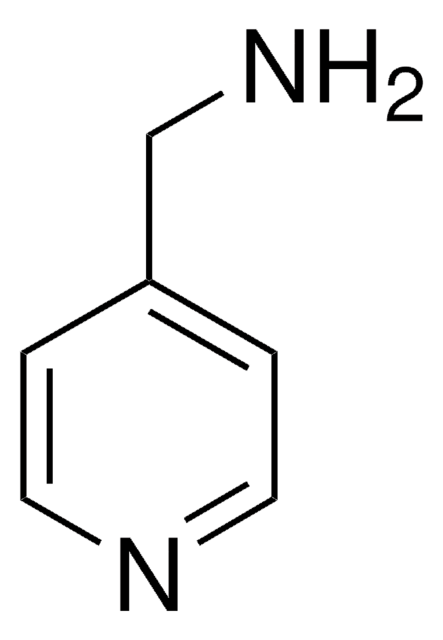
Empirical Formula (Hill Notation):
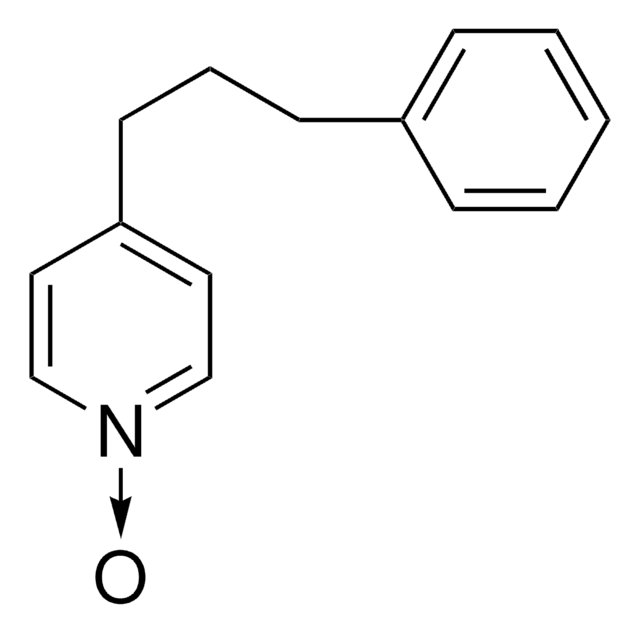
C14H15N
CAS Number:
Molecular Weight:
197.28
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39151717
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
refractive index
n20/D 1.563 (lit.)
bp
322 °C (lit.)
solubility
H2O: insoluble
density
1.03 g/mL at 25 °C (lit.)
functional group
phenyl
SMILES string
C(Cc1ccccc1)Cc2ccncc2
InChI
1S/C14H15N/c1-2-5-13(6-3-1)7-4-8-14-9-11-15-12-10-14/h1-3,5-6,9-12H,4,7-8H2
InChI key
AQIIVEISJBBUCR-UHFFFAOYSA-N
General description
4-(3-Phenylpropyl) pyridine is an organic solvent and is a suitable medium for water insoluble transition metal complexes of porphyrins or phthalocyanines.
Application
4-(3-Phenylpropyl) pyridine(PPP) was used in electrochemical processes for a novel system of microdroplets of PPP immobilised at graphite and in mesoporous ceramic electrodes. PPP has been used to investigate electrochemically driven mono-, di-, and tri-carboxylate anion transfer at the 4-(3-phenylpropyl)pyridine/aqueous electrolyte interface for a triple phase boundary system at graphite electrodes.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Frank Marken et al.
Faraday discussions, 129, 219-229 (2005-02-18)
Biphasic electrode systems allow electrochemical reactions to be driven in a microphase of organic liquid (typically 1-100 nL), which is coupled via ion transfer processes to the surrounding aqueous electrolyte medium. Microdroplet deposits on basal plane pyrolytic graphite as well
Stuart M MacDonald et al.
Physical chemistry chemical physics : PCCP, 10(26), 3925-3933 (2008-08-09)
Understanding liquid|liquid ion transfer processes is important in particular for naturally occurring species such as carboxylates. In this study electrochemically driven mono-, di-, and tri-carboxylate anion transfer at the 4-(3-phenylpropyl)pyridine|aqueous electrolyte interface is investigated experimentally for a triple phase boundary
Xueqiang Qi et al.
Dalton transactions (Cambridge, England : 2003), 43(24), 9283-9295 (2014-05-14)
Core-shell RuPt (Ru core-Pt shell) and PtRu (Pt core-Ru shell) nanoparticles were prepared by decomposing in a two-step procedure a ruthenium ([Ru(COD)(COT)] (COD = 1,5-cyclooctadiene, COT = 1,3,5-cyclooctatriene)) and a platinum complex ([Pt2(dba)3] (dba = dibenzylideneacetone) or [Pt(CH3)2(COD)]) in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![4-[6-(4-pyridinyl)hexyl]pyridine AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/969/304/69102a98-4497-4db9-b5d7-792b9c6fb5e7/640/69102a98-4497-4db9-b5d7-792b9c6fb5e7.png)