112372
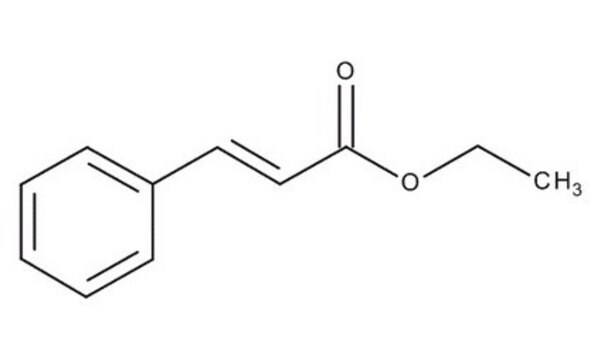
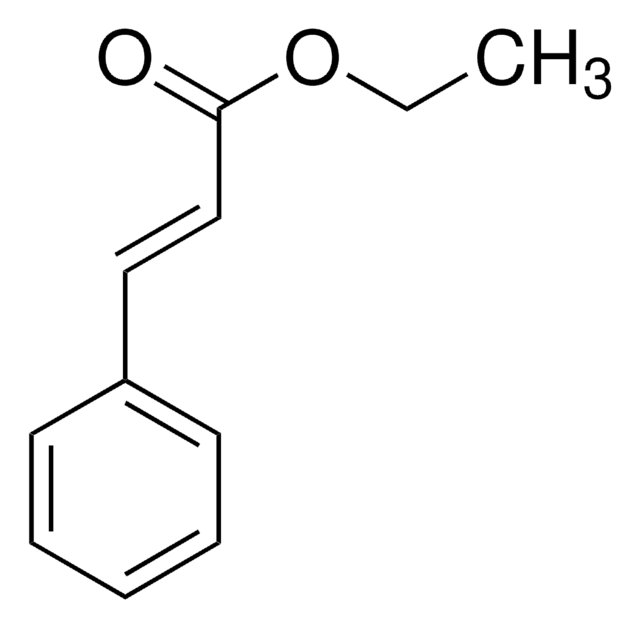
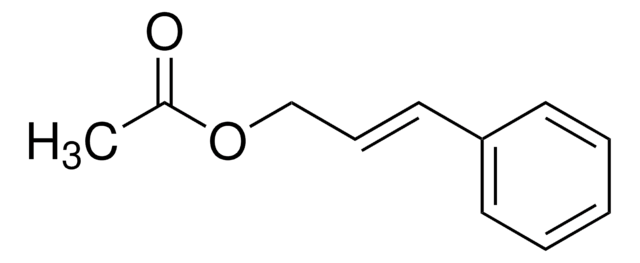
Ethyl cinnamate
99%
Synonym(s):
3-Phenyl-2-propenoic acid ethyl ester, Ethyl 3-phenyl-2-propenoate, NSC 6773
About This Item
Recommended Products
assay
99%
impurities
may contain alpha-tocopherol (synthetic)
refractive index
n20/D 1.558 (lit.)
bp
271 °C (lit.)
mp
6-8 °C (lit.)
density
1.049 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
CCOC(=O)\C=C\c1ccccc1
InChI
1S/C11H12O2/c1-2-13-11(12)9-8-10-6-4-3-5-7-10/h3-9H,2H2,1H3/b9-8+
InChI key
KBEBGUQPQBELIU-CMDGGOBGSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Antimicrobial and antiproliferative effects: Essential oils including Ethyl cinnamate derived from Zingiberaceae family show promising antimicrobial and antiproliferative properties, suggesting their potential application in oral healthcare products. This study provides insight into the utilization of natural products for medical and pharmaceutical applications (Amil et al., 2024).
- Biodistribution and cellular interactions in medical research: Ethyl cinnamate could be investigated for its role in enhancing the permeability and imaging contrast in studies like those investigating the biodistribution of intravenously delivered mesenchymal stromal cells. Such applications could improve understanding in cellular therapies and regenerative medicine (Pichardo et al., 2022).
Biochem/physiol Actions
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service