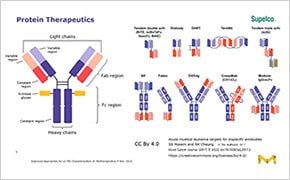
Biopharmaceutical Characterization

Biopharmaceuticals are medical drugs produced using biotechnological methods. These include monoclonal antibodies (mAbs), therapeutic proteins, fusion proteins, antibody drug conjugates, and other such biologics. Characterization testing is an understanding of the physical and chemical properties of biopharmaceutical materials. During drug development, these properties can have an impact on the product’s performance, ability to be processed, stability and appearance.
Biologics, biosimilars characterization and analysis
Biologic drugs require highly sophisticated analytical workflows for their analysis and characterization with significant emphasis on GMP and regulatory compliance. Development of both an original biotherapy or a biosimilar requires product characterization studies to ensure a robust and well specified biological drug.
Read more about
Related Technical Articles
- A complete SEC-UV workflow for the characterization of mAb monomers, aggregates, and fragments using Zenix® and Zenix®-C SEC columns, including system suitability testing and forced pH and temperature stress studies.
- Optimization of a Reversed-Phase Liquid Chromatographic (RP-LC) method for the intact analysis of a therapeutic monoclonal antibody, trastuzumab, using a BIOshell™ A400 Protein C4 column.
- Explore various strategies for deglycosylating N-linked glycans involving PNGase F, PNGase A (Glycopeptidase A), and even native and sequential deglycosylation with endoglycosidases like Endoglycosidase H, Endoglycosidase F, and exoglycosidases.
- Comparative analysis of different columns in resolving medium-sized fragments of monoclonal antibodies, after digestion using dithiothreitol (DTT) or IdeS (a protease), by Reversed-Phase Chromatography.
- Step-by-step workflows for the intact mass analysis, peptide mapping, and N-glycan analysis of the monoclonal antibody― adalimumab, for an accurate characterization of the critical quality attributes (CQAs) to ensure drug safety and efficacy. Read more.
- See All (17)
Related Protocols
- An optimized LC-MS/MS based workflow for low artifact tryptic digestion and peptide mapping of monoclonal antibody, adalimumab (Humira) using filter assisted sample preparation (FASP).
- A step-by-step protocol for released N-linked glycan analysis of the monoclonal antibody adalimumab, based on UHPLC-FLR-MS and procainamide labeling.
- A complete workflow for the intact and middle-up mass analysis of reduced and non-reduced monoclonal antibodies based on SEC-MS with sample preparation by protein-A affinity clean-up.
- BIOshell™ IgG 1000 Å C4 UHPLC Column for Improved Biomacromolecule Separations
- SigmaMab Antibody Drug Conjugate Mimic, is a non-toxic drug mimic utilized as a standard for mass spectrometry and high performance liquid chromatography.
- See All (5)
Find More Articles and Protocols
Related Resources
- Brochure: A Quick Guide to Successful Dissolution Testing
From development to drug release, this interactive PDF outlines a range of reliable, consistent filters and reagents for dissolution testing protocol.
- Analytix Reporter: Pharma Analysis & QC
This series of technical articles of the Analytix Reporter special edition outlines biologics characterization being performed in the LC-MS lab.
- Guide: Biopharma Analysis
This interactive PDF details the characterization and analysis of various biopharmaceutical modalities, from antibodies to oligonucleotides and beyond.
Identity
Intact Mass Determination
Determination of the intact molecular mass, often performed with SEC-MS and appropriate standards, is a necessary step in the characterization of biologics, from clone selection to verification of the final product. Intact molecular mass analysis is commonly used to demonstrate the diversity of the protein/peptide products and verify the identity of the biologics. With optimization, it can also determine the intact molecular weight of all protein products, including bispecific monoclonal antibodies.
Instead of dealing with the unwieldly intact mass of a large protein biologic, a protein digestion analysis approach is used where the sample is first cleaved into just a few fragments, following which the separation can be handled individually with specialized mass spec proteomic standards.
Titer Measurement
Productivity of the cell line to deliver sufficient quantities of mAb, influences its commercial potential. Used as a baseline measurement, titer is measured using Protein-A affinity chromatography (HPLC). Initially in the development of a mAb, a large number of harvest cell culture (HCC) samples were to be screened for IgG titer. Affinity chromatography employing a Protein A ligand is often used to determine the mAb concentration as well as to purify it for downstream aggregate and charge variant analysis.
Amino Acid Analysis
Amino acid analysis is a popular way to establish product identity and is used in the determination of amino acid composition of a mAb. It is often performed alongside an extinction coefficient measurement, a method routinely employed to establish titer between different productions.
Certified Reference Materials with values assigned by metrologically valid procedures will be critical to minimize and control experimental variations in all steps of the amino acid analysis workflow including protein extraction, fractionation, enrichment, proteolysis, and analysis.
Peptide Mapping
To gain an indication of the identity of the mAb, comparison of simple, single enzyme peptide maps may be sufficient. More detailed therapeutic antibody characterization, including N- or C-terminal sequencing, glycan characterization, and other aspects of antibody production, analysis, purification, and fragmentation can better inform product engineering. Proteomic mass spectrometry provides additional structural information.
Post-translational Modification
N-glycan Analysis
Highly variable glycosylation may impact mAb purity and produce variable function. Monoclonal antibodies carry N-glycans on their backbone that can be released using LC-MS to assess glycosylation patterns. Characterization of the N-glycans from a monoclonal antibody is necessary, to provide full structural details of the molecule under investigation. Understanding these N-glycan pathways is important because N-glycans affect many properties of glycoproteins including their conformation, solubility, antigenicity, and recognition by glycan-binding proteins.
Monoclonal Antibodies (mAbs) Higher Order Structure (HOS) Comparison
Many factors influence HOS – the 3D structure of a mAb – ranging from the choice of cell line for mAb production to bioprocessing conditions such as temperature, pH, and light exposure. Hydrogen deuterium exchange mass spectrometry (HDX-MS) is a method employed for a detailed insight into the tertiary structure of the mAb.
mAb Modification Analysis
Many different post-translational modifications can occur during the mAb manufacturing process, which are greatly influenced by process parameters such as media, temperature, etc. To produce a consistent product, it is critical these modifications are reproduced during every round of mAb synthesis. Modification analysis includes disulfide bridge mapping, and evaluation of glycan basic structure. It is also wise to quantify sialylation, as sialic acid can have a negative effect on mAb.
mAb Charge Distribution
As a result of post-translational modifications (PTMs) or chemical modifications, charge variants can have a considerable impact on the biological activity and pharmacokinetics of a mAb. A regulatory requirement for mAbs, charge variant analysis can be evaluated by techniques including cation exchange chromatography (CEX) and capillary isoelectric focusing (cIEF).
mAb Physical Testing & Size Distribution
mAb Physical Testing
Physical testing is used to characterize the appearance of a mAb, for example measurement of pH, osmolality, and concentration. Packaging integrity is also assessed within physical testing protocol, for example using Karl Fischer moisture analysis or dye ingress to confirm closure integrity.
HmAb Size Distribution
While a single mAb product is desired, the initial production material often contains size variants such as aggregates, fragments, and biomolecules exhibiting additional light chains. Since these have the potential to affect immunogenicity and potency, it is important to monitor their presence. Size exclusion chromatography (SEC) is the most commonly used method to evaluate size distribution.
Sterility & Impurities
Host-cell Protein Impurities
Host cell protein (HCP) impurities, present at PPM-levels in biotherapies, are a major immunogenicity risk, as they can elicit an unpredictable immune response in patients. Their complex and diverse nature makes them challenging to detect or monitor. While most HCP impurities are effectively removed in typical downstream purification processes, a small population of HCPs are particularly challenging. Knockouts of a difficult-to-remove CHO HCP, lipoprotein lipase have been developed for improved polysorbate stability in monoclonal antibody formulations.
Manufacturing Additives Monitoring
A wide variety of manufacturing additives must be monitored during mAb development and manufacturing including Detergents, Protein A, Transfection reagents, Antibiotics, Anti-foam agents, and Growth factors.
mAbs Microbial Testing
A variety of microbial testing procedures are necessary for GMP and compliant pharmaceutical development and manufacturing. Many mAbs are produced in microorganisms to benefit from their rapid growth rates and high yields, making it critical to monitor and control the presence of microbial contaminants. A component of the cell wall of Gram-negative bacteria, called endotoxin, produces responses ranging from fever and chills to fatal septic shock, making pyrogen detection (MAT in vitro testing) and subsequent removal from the mAb crucial and a regulatory requirement. Bioburden testing should be employed throughout the entire mAb manufacturing process to monitor the presence of potentially harmful microbial contamination. Sterility testing is also critical to confirm the integrity of mAb production.
Related Webinars
This webinar will cover basic chromatographic approaches to intact and middle-up analysis of mAbs before delving into more detailed and complex strategies for analysis.
Webinar: Tips for Improved Peptide Mapping
This webinar discusses advances in “bottom-up” analysis of monoclonal antibodies, while highlighting the role and importance column chemistry still plays in developing highly selective high-performance liquid chromatography (HPLC) methods for peptides.
This webinar will show how to enhance understanding of improving precision in biotherapeutic characterization through innovative methodologies.
To continue reading please sign in or create an account.
Don't Have An Account?