Wszystkie zdjęcia(1)
Kluczowe dokumenty
F8682
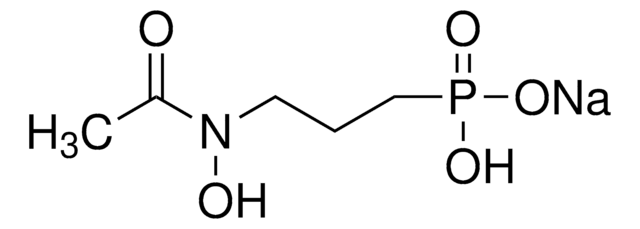
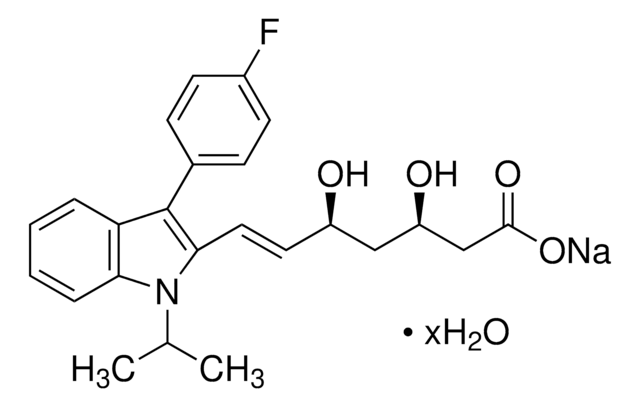
Fosmidomycin sodium salt hydrate
≥95% (NMR)
Synonim(y):
(3-(Formylhydroxyamino)propyl)phosphonic acid sodium salt, (3-(N-Hydroxyformamido)propyl)phosphonic acid sodium salt, FR 31564
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C4H10NO5P · xNa+ · yH2O
Numer CAS:
Masa cząsteczkowa:
183.10 (anhydrous free acid basis)
Numer MDL:
Kod UNSPSC:
12352200
NACRES:
NA.77
Polecane produkty
Poziom jakości
Próba
≥95% (NMR)
Formularz
powder
warunki przechowywania
desiccated
kolor
white to beige
rozpuszczalność
H2O: 20 mg/mL, clear
temp. przechowywania
−20°C
ciąg SMILES
[P](=O)(O)(O)CCCN(O)C=O
InChI
1S/C4H10NO5P/c6-4-5(7)2-1-3-11(8,9)10/h4,7H,1-3H2,(H2,8,9,10)
Klucz InChI
GJXWDTUCERCKIX-UHFFFAOYSA-N
Powiązane kategorie
Zastosowanie
Fosmidomycin sodium salt hydrate has been used as an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in a study to determine monotropin carvacrol biosynthesis in Satureja khuzistanica plant.
Działania biochem./fizjol.
Fosmidomycin is an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) (MEP synthase): an antimalarial compound.
Fosmidomycin is an inhibitor of 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) (MEP synthase): an antimalarial compound. 1-deoxy-D-xylulose 5-phosphate reductoisomerase (DXR) is an enzyme involved in the first step in the nonmevalonate pathway for isoprenoid biosynthesis in Gram-negative, Gram-positive bacteria, plants, and the parasite causing the most virulent form of malaria, Plasmodium falciparum (Mammals produce isoprenoids via the mevalonate pathway).
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Karin Brücher et al.
Journal of medicinal chemistry, 55(14), 6566-6575 (2012-06-27)
Specific inhibition of enzymes of the non-mevalonate pathway is a promising strategy for the development of novel antiplasmodial drugs. α-Aryl-substituted β-oxa isosteres of fosmidomycin with a reverse orientation of the hydroxamic acid group were synthesized and evaluated for their inhibitory
Christoph T Behrendt et al.
Journal of medicinal chemistry, 54(19), 6796-6802 (2011-08-27)
Reverse hydroxamate-based inhibitors of IspC, a key enzyme of the non-mevalonate pathway of isoprenoid biosynthesis and a validated antimalarial target, were synthesized and biologically evaluated. The binding mode of one derivative in complex with EcIspC and a divalent metal ion
Emily R Jackson et al.
Current topics in medicinal chemistry, 12(7), 706-728 (2012-01-31)
Isoprene biosynthesis is an essential component of metabolism. Two pathways are known for the production of five-carbon (isoprene) intermediates: the mevalonate and nonmevalonate pathways. As many pathogenic organisms rely exclusively on the nonmevalonate pathway (NMP) for isoprenoids and humans do
Robert R Junker et al.
Journal of chemical ecology, 37(12), 1323-1331 (2011-12-14)
In their natural environment, plants are synchronously confronted with mutualists and antagonists, and thus benefit from signals that contain messages for both functional groups of interaction partners. Floral scents are complex blends of volatiles of different chemical classes, including benzenoids
Catherine Zinglé et al.
Bioorganic & medicinal chemistry letters, 22(21), 6563-6567 (2012-10-03)
Fosmidomycin derivatives in which the hydroxamic acid group has been replaced by several bidentate chelators as potential hydroxamic alternatives were prepared and tested against the DXR from Escherichia coli. These results illustrate the predominant role of the hydroxamate functional group
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej