E9906
Anti-EDEM2 (N-terminal) antibody produced in rabbit
~1.0 mg/mL, affinity isolated antibody, buffered aqueous solution
Synonim(y):
Anti-ER degradation enhancer, mannosidase alpha-like 2
About This Item
Polecane produkty
pochodzenie biologiczne
rabbit
białko sprzężone
unconjugated
forma przeciwciała
affinity isolated antibody
rodzaj przeciwciała
primary antibodies
klon
polyclonal
Postać
buffered aqueous solution
masa cząsteczkowa
antigen ~70 kDa
reaktywność gatunkowa
human, rat (predicted), mouse
stężenie
~1.0 mg/mL
metody
indirect immunofluorescence: 5-10 μg/mL using mouse 3T3 cells
western blot: 0.5-1 μg/mL using whole extracts of HEK-293T cells expressing recombinant human EDEM2
numer dostępu UniProt
Warunki transportu
dry ice
temp. przechowywania
−20°C
docelowa modyfikacja potranslacyjna
unmodified
informacje o genach
human ... EDEM2(55741)
mouse ... Edem2(108687)
rat ... Edem2(296304)
Opis ogólny
Immunogen
Zastosowanie
Działania biochem./fizjol.
Postać fizyczna
Oświadczenie o zrzeczeniu się odpowiedzialności
Nie możesz znaleźć właściwego produktu?
Wypróbuj nasz Narzędzie selektora produktów.
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
nwg
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej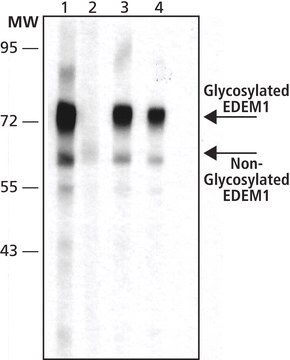
![(±)-(E)-4-Ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide ≥98%](/deepweb/assets/sigmaaldrich/product/structures/404/859/0c6eb80d-2c3c-4bf1-98e8-1b319b597b12/640/0c6eb80d-2c3c-4bf1-98e8-1b319b597b12.png)