Key Documents
Y0000408
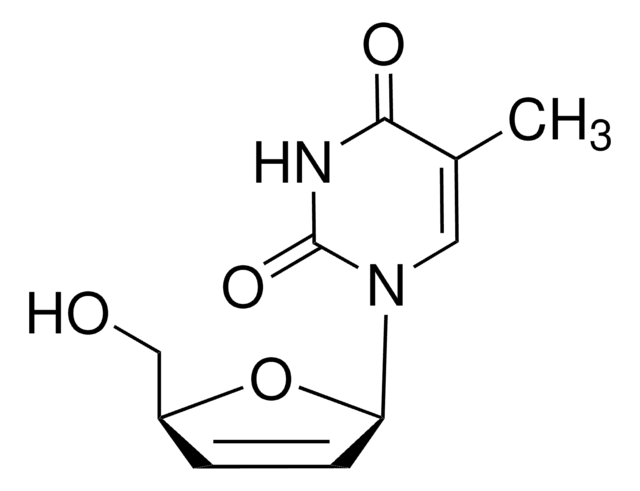
Stavudine
European Pharmacopoeia (EP) Reference Standard
Synonim(y):
2′,3′-Didehydro-3′-deoxythymidine, 1-(2,3-Dideoxy-β-D-glycero-pent-2-enofuranosyl)thymine, 1-[(2R,5S)-5-(Hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methylpyrimidine-2,4-(1H,3H)-dione, 2′,3′-Anhydrothymidine, Stavudine, d4T
About This Item
Polecane produkty
klasa czystości
pharmaceutical primary standard
rodzina API
stavudine
producent / nazwa handlowa
EDQM
Zastosowanie
pharmaceutical (small molecule)
format
neat
temp. przechowywania
2-8°C
ciąg SMILES
CC1=CN([C@@H]2O[C@H](CO)C=C2)C(=O)NC1=O
InChI
1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1
Klucz InChI
XNKLLVCARDGLGL-JGVFFNPUSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Opakowanie
Inne uwagi
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej