Kluczowe dokumenty
37018
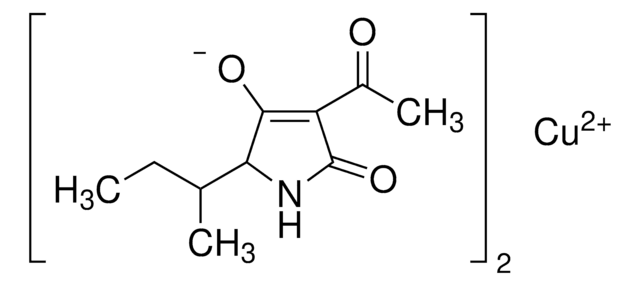
Tenuazonic acid
analytical standard
Synonim(y):
(5S)-3-Acetyl-1,5-dihydro-4-hydroxy-5-[(1S)-1-methylpropyl]-2H-pyrrol-2-one, (S)-3-Acetyl-5-(S)-sec-butyltetramic acid, (S)-3-Acetyl-5-sec-butyl-4-hydroxy-3-pyrrolin-2-one
About This Item
Polecane produkty
klasa czystości
analytical standard
Poziom jakości
okres trwałości
limited shelf life, expiry date on the label
metody
HPLC: suitable
gas chromatography (GC): suitable
Zastosowanie
cleaning products
cosmetics
food and beverages
personal care
Format
neat
temp. przechowywania
−20°C
ciąg SMILES
CC[C@H](C)[C@@H]1NC(=O)C(C(C)=O)=C1O
InChI
1S/C10H15NO3/c1-4-5(2)8-9(13)7(6(3)12)10(14)11-8/h5,8,13H,4H2,1-3H3,(H,11,14)/t5-,8-/m0/s1
Klucz InChI
CEIZFXOZIQNICU-XNCJUZBTSA-N
Opis ogólny
Zastosowanie
- Cornflakes using high-performance liquid chromatography (HPLC) technique.
- Human urine samples using isotope dilution assay method and liquid chromatography coupled to a hybrid triple quadrupole/linear ion trap mass spectrometer.
- Cereals using high-performance liquid chromatography–electrospray ionization ion-trap multistage mass spectrometry (HPLC- ESI ion-trap (IT) MS2). ESI Fourier transform-ion cyclotron resonance tandem mass spectrometry (FTICR-MS2) technique is used for product characterization, post derivatization with 2,4-dinitrophenylhydrazine.
Rekonstytucja
Komentarz do analizy
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Oral
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej