Kluczowe dokumenty
06709
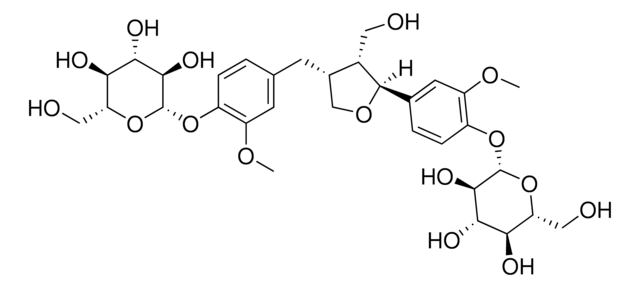
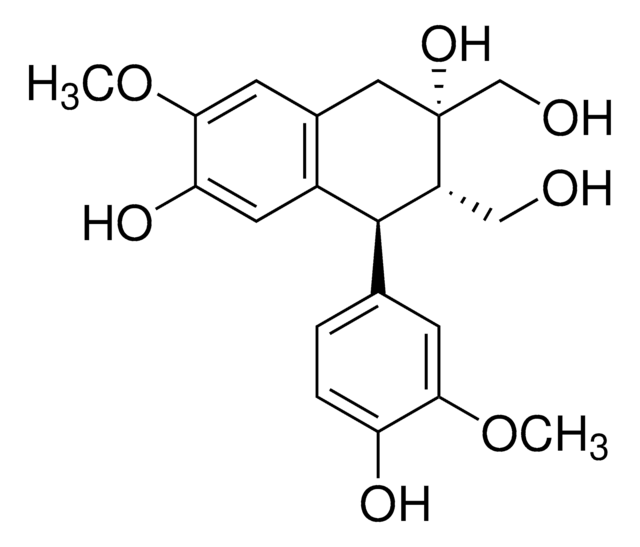
Pinoresinol
analytical standard
Synonim(y):
(+)-Pinoresinol, 4,4′-((1S,3aR,4S,6aR)-Hexahydrofuro[3,4-c]furan-1,4-diyl)bis(2-methoxyphenol), 4,4′-[(1S,3aR,4S,6aR)-Tetrahydro-1H,3H-furo[3,4-c]furan-1,4-diyl]bis(2-methoxyphenol)
About This Item
Polecane produkty
Poziom jakości
klasa czystości
analytical standard
Próba
≥95.0% (HPLC)
okres trwałości
limited shelf life, expiry date on the label
metody
HPLC: suitable
gas chromatography (GC): suitable
Zastosowanie
cleaning products
cosmetics
food and beverages
personal care
format
neat
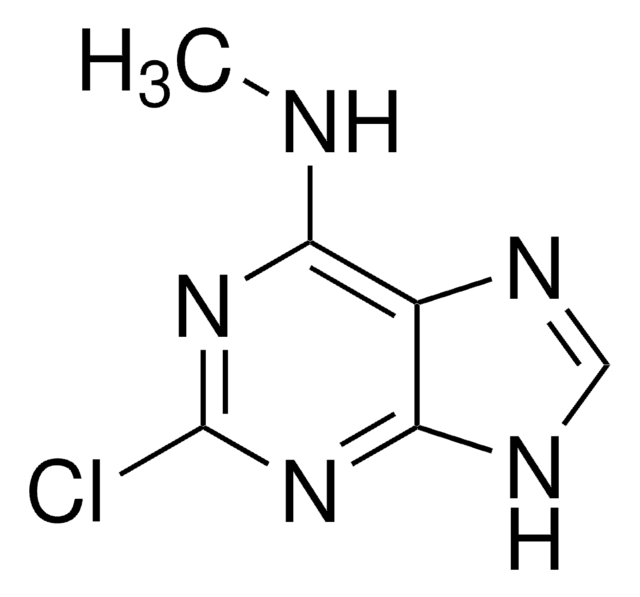
ciąg SMILES
COc1cc(ccc1O)[C@H]2OC[C@H]3[C@@H]2CO[C@@H]3c4ccc(O)c(OC)c4
InChI
1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19+,20+/m0/s1
Klucz InChI
HGXBRUKMWQGOIE-AFHBHXEDSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Działania biochem./fizjol.
Opakowanie
Polecane produkty
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej