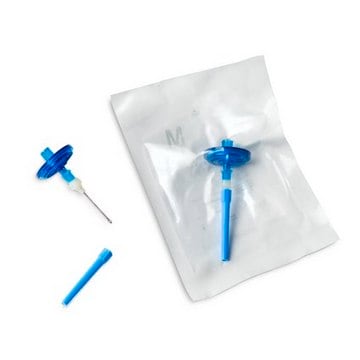
TZHVLV205
Steritest® NEO Device
For liquids in large vials. Red base canister with a vented double needle for large glass containers with septa. Double packed.
Synonim(y):
Red Base Steritest® NEO device for sterility testing, Sterility testing device, membrane filtration device, membrane filtration canister, closed membrane filtration
About This Item
Polecane produkty
Materiały
Nylon 66 adapter (for needle)
PVC tubing (double lumen)
PVDF membrane
plain filter
stainless steel (for needle)
styrene-acrylonitrile (SAN) (for canister)
Poziom jakości
agency
EP (2.6.1)
JP (4.06)
USP 71
sterylność
sterile; γ-irradiated
producent / nazwa handlowa
Steritest®
opakowanie
pkg of 10 blisters in 2 bags of 5 blisters per box, Double packed
Parametry
120 mL sample volume (graduation marks at 25, 50, 75 and 100 mL)
3.1 bar max. inlet pressure (45 psi) at 25 °C
45 °C max. temp.
dł. rurki
850 mm
kolor
red Canister Base
macierz
Durapore®
wielkość porów
0.45 μm pore size
moc wejściowa
liquid
sample type pharmaceutical(s)
Zastosowanie
pharmaceutical
sterility testing
Warunki transportu
ambient
Powiązane kategorie
Opis ogólny
Steritest® NEO is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system ensures that pharmaceutical products are never exposed to the environment, minimizes false positives, and offers the highest levels of quality & reliability. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The Steritest® NEO Double Packed Device for liquids in large vials is gamma sterilized and double packed for the quick transfer into sterility testing environments, simplifying decontamination procedures and saving time. A vented needle adapter vents and transfers the test product from large volume containers with a septum to the Steritest® NEO devices. The red canister base indicates low absorption. Durapore® hydrophilic Poly vinylidene fluoride (PVDF) membrane and specific drain design. This optimizes the rinsing of products that inhibit microbial growth.
Zastosowanie
Cechy i korzyści
- One-stop-shop for sterility testing with our devices, pumps, media, fluids, and services
- Steritest® devices are manufactured in our Center of Excellence in Molsheim, France, with high-quality control standards maintaining the Certificate of Quality for each lot.
- New needle design
- Smarter workflow
- Completely closed set up
- Consistent performance
- New tubing disconnection tool
Opakowanie
Informacje prawne
skonfigurowane dla
Kod klasy składowania
10-13 - German Storage Class 10 to 13
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Produkty
Steritest™ NEO System – Specific Steritest™ NEO closed systems allow aseptic dissolution/dilution of product samples. The Steridilutor® NEO system is specifically designed to dissolve and dilute drugs in vials. The Steridilutor® NEO device for liquid transfer kit enables to dilute liquids from open ampoules into a diluent container with septum.
Testowanie sterylności jest jednym z najważniejszych etapów uwalniania produktów farmaceutycznych. Zgodne z przepisami urządzenia do testowania sterylności metodą filtracji membranowej zapewniają bezpieczeństwo produktów farmaceutycznych.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej