Opis ogólny
Phosphorylation plays an important role in regulating protein activities and various cellular signaling events in cells. Limited by the tools available for phosphohistidine (pHis) detection, the majority of studies focus on serine, threonine, and tyrosine phosphorylations. Histidine phosphorylation can occur at either N1 (1-pHis) or N3 (3-pHis) of the imidazole ring. The development of peptides containing stable phosphoryltriazolylalanine analogues of 1-pHis and 3-pHis (1-pTza and 3-pTza) allows the generation of antibodies for studying both histidine N1 and N3 phosphorylations in signaling events. There is growing evidence implicating His kinases in cancer and tumor metastasis and the first metastasis suppressor gene identified is one of the two known mammalian His kinases, Nm23-H1 (also known as NME1, nucleoside diphosphate kinase, or NDPK-A). Nm23-H1/NME1 and the closely related Nm23-H2 (NME2/NDPK-B) catalyze the transfer of phosphate from ATP onto Nucleoside-diphosphates (NDPs) through a 1-pHis enzyme intermediate. Nm23-H1/-H2 also possess His kinase activity, transferring the phosphate from the active site pHis onto a His in a target protein. Metabolic enzymes such as phosphoglycerate mutase (PGAM), succinyl CoA synthase (SCS), and ATP citrate lyase (ACL) also use pHis as an enzyme intermediate. Unlike NME1/2, PGAM uses 3-pHis as an enzyme intermediate. In addition to eukaryotes, histidine phosphorylation is well documented in bacterial “two-component” signaling pathways involved in chemotaxis, although the phosphate is transferred from the pHis formed in the receptor/sensor protein to Asp residues of an acceptor response regulator protein, and the receptor/sensor protein essentially functions as an aspartate kinase.
Specyficzność
Selectively detects proteins with histidine(s) phosphorylated at N3 of the imidazole ring (3-pHis), but not 1-pHis.
Target modification is not species specific.
Immunogen
Epitope: N3-phosphohistidine (3-pHis)
KLH-conjugated library of random peptides containing non-hydrolyzable phosphohistidine analogue 3-pTza.
Zastosowanie
Anti-N3-Phosphohistidine (3-pHis) antibody, clone SC39-6 is an isomer-specific monoclonal Ab to specifically detect histidine phosphorylated at position N3. This purified mAb is backed by published data demonstrating performance in Western blotting.
Note: DO NOT HEAT SAMPLES prior to phosphohistidine detection. Histidine phosphorylation is heat and acid labile. To generate negative control for specificity test, an aliquot of sample can be heated at 95ºC for 10-15 minutes to reverse histidine phosphorylation. Alternatively, an aliquot of sample can be incubated under acidified pH at 37ºC for 15 minunites to reduce histidine phosphorylation. Acidify each 100 µL sample with 25 µL of 1 M HCl before the incubation, then neutralize with 25 µL of 1 M NaOH prior to phosphohistidine detection.
Research Category
Signaling
Research Sub Category
Signaling Neuroscience
Jakość
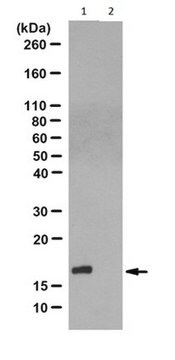
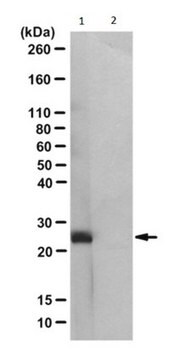
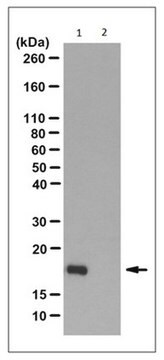
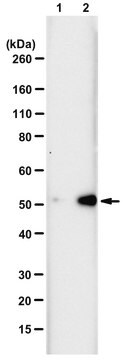
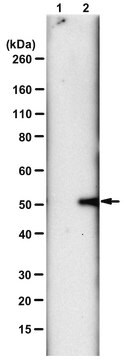
Evaluated by Western Blotting of PGAM-catalyzed 2,3-DPG degradation reaction.
Western Blotting Analysis: 0.08 µg/mL of this antibody detected recombinant human phosphoglycerate mutase (PGAM) with N3-phosphohistidine (3-pHis) in a 5 µg aliquot of PGAM-catalyzed 2,3-diphosphoglycerate (2,3-DPG) degradation reaction.
Opis wartości docelowych
Variable depending on the histidine-phosphorylated proteins.
Postać fizyczna
Format: Purified
Protein A purified
Purified rabbit monoclonal antibody in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide.
Przechowywanie i stabilność
Stable for 1 year at 2-8°C from date of receipt.
Inne uwagi
Concentration: Please refer to lot specific datasheet.
Oświadczenie o zrzeczeniu się odpowiedzialności
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Ta strona może zawierać tekst przetłumaczony maszynowo.