Kluczowe dokumenty
569755
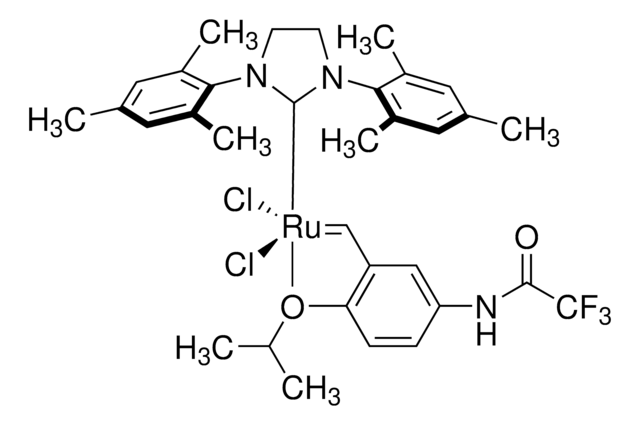
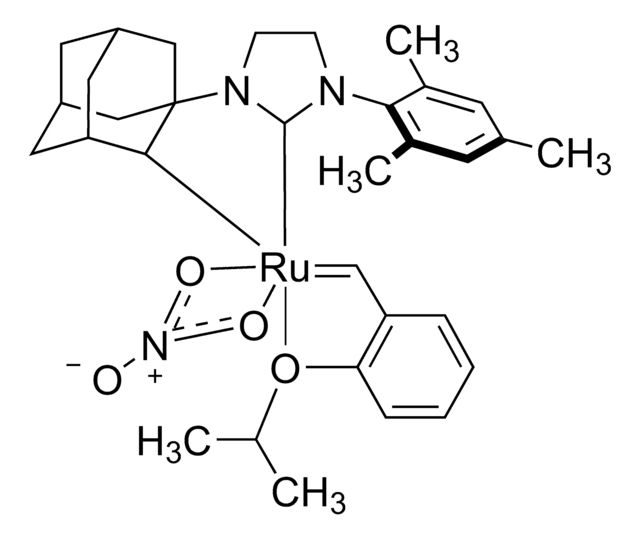
Hoveyda-Grubbs Catalyst® M720
Umicore, 97%
Synonim(y):
Hoveyda-Grubbs Catalyst® 2nd Generation, Hoveyda-Grubbs Catalyst® M72 (C627), (1,3-Bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(o-isopropoxyphenylmethylene)ruthenium, Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene](2-isopropoxyphenylmethylene)ruthenium(II), Grubbs Catalyst® C627
About This Item
Polecane produkty
Poziom jakości
Próba
97%
Formularz
solid
przydatność reakcji
core: ruthenium
reagent type: catalyst
reaction type: Ring-Opening Polymerization
mp
216-220 °C (lit.)
temp. przechowywania
2-8°C
ciąg SMILES
CC(C)Oc1ccccc1C=[Ru](Cl)(Cl)=C2N(CCN2c3c(C)cc(C)cc3C)c4c(C)cc(C)cc4C
InChI
1S/C21H26N2.C10H12O.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-8(2)11-10-7-5-4-6-9(10)3;;;/h9-12H,7-8H2,1-6H3;3-8H,1-2H3;2*1H;/q;;;;+2/p-2
Klucz InChI
ZRPFJAPZDXQHSM-UHFFFAOYSA-L
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Zastosowanie
It can be used as a catalyst for olefin cross-metathesis with fluorinated olefins (CM), ring-closing metathesis (RCM), ring-opening metathesis (ROM), and a sequence of a metathesis reaction and subsequent dihydroxylation of the newly formed double bond.
Learn more about our metathesis catalysts
Informacje prawne
Product License
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
produkt powiązany
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej





![Dichloro[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene][[5-[(dimethylamino)sulfonyl]-2-(1-methylethoxy-O)phenyl]methylene-C]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/179/573/f48a2a1e-cf09-4151-8b78-2bab614efd5c/640/f48a2a1e-cf09-4151-8b78-2bab614efd5c.png)
![2,6-Diisopropylphenylimido-neophylidene[(S)-(−)-BIPHEN]molybdenum(VI) ringclosing metathesis catalyst, ≥95.0% (C)](/deepweb/assets/sigmaaldrich/product/structures/312/745/96ea840b-77a7-427a-9db5-fa08b3ffd45e/640/96ea840b-77a7-427a-9db5-fa08b3ffd45e.png)