S8160
Superoxide Dismutase from bovine liver
lyophilized powder, ≥1500 units/mg protein
Synonym(s):
SOD, Superoxide: superoxide oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥1500 units/mg protein
mol wt
32.5 kDa
composition
Protein, ≥70% biuret
UniProt accession no.
storage temp.
−20°C
Gene Information
cow ... SOD1(281495)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
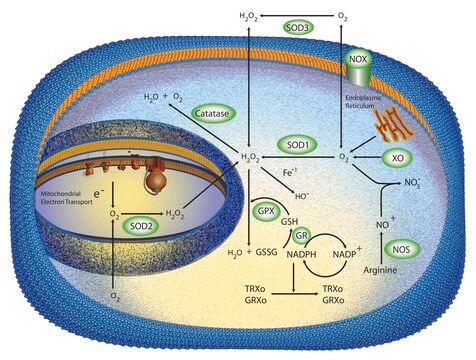
Research area: Cell signalingSuperoxide Dismutase (SOD), a low molecular weight protein that is found in all aerobic cells of microorganisms, plants, and animals. It exists as three distinct families such as manganese SOD, copper–zinc SOD, and extracellular SOD.
Application
Superoxide dismutase from bovine liver has been used in a study to determine that hypercholesterolemia increases endothelial superoxide anion production. Superoxide dismutase from bovine liver has also been used in a study to investigate diazo coupling, subunit interactions, and electrophoretic variants of bovine erythrocyte superoxide dismutase. It has also been used as a component of the assay buffer in thequantification of reactive oxygen species (ROS) by O2k fluorometry.
Biochem/physiol Actions
Superoxide Dismutase catalyzes the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen. It plays a critical role in the defense of cells against the toxic effects of oxygen radicals. It competes with nitric oxide (NO) for superoxide anion (which reacts with NO to form peroxynitrite), thereby SOD promotes the activity of NO. SOD has also been shown to suppress apoptosis in cultured rat ovarian follicles, neural cell lines, and transgenic mice.
Unit Definition
One unit will inhibit reduction of cytochrome c by 50% in a coupled system with xanthine oxidase at pH 7.8 at 25 °C in a 3.0 mL reaction volume. Xanthine oxidase concentration should produce an initial ΔA550 of 0.025 ± 0.005 per min.
Physical form
Lyophilized powder containing potassium phosphate buffer salts
Analysis Note
For assay method, see McCord, J.M. and Fridovich, I., J. Biol. Chem., 244, 6049 (1969).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S D Yan et al.
The Journal of biological chemistry, 269(13), 9889-9897 (1994-04-01)
Attack by reactive oxygen intermediates, common to many kinds of cell/tissue injury, has been implicated in the development of diabetic and other vascular diseases. Such oxygen-free radicals can be generated by advanced glycation end products (AGEs), which are nonenzymatically glycated
Bovine erythrocyte superoxide dismutase: diazo coupling, subunit interactions, and electrophoretic variants.
D P Malinowski et al.
Biochemistry, 18(1), 237-244 (1979-01-09)
Y Ohara et al.
The Journal of clinical investigation, 91(6), 2546-2551 (1993-06-01)
Indirect evidence suggests accelerated degradation of endothelium-derived nitric oxide (ENDO) by superoxide anion (O2-) in hypercholesterolemic vessels (HV). To directly measure O2- production by normal vessels (NV) and HV, we used an assay for O2- based on the chemiluminescence (CL)
Luciana Cacciottola et al.
Fertility and sterility, 110(3), 534-544 (2018-07-02)
To characterize oxidative stress and metabolic activity in xenografted human ovarian tissue using microdialysis. Prospective experimental study. Gynecology research unit at a university hospital. Cryopreserved ovarian cortex from five women 27-35 years of age. Frozen-thawed human ovarian tissue fragments were xenografted
Naoya Ichimaru et al.
Biochemistry, 47(40), 10816-10826 (2008-09-11)
The mode of action of Deltalac-acetogenins, strong inhibitors of bovine heart mitochondrial complex I, is different from that of traditional inhibitors such as rotenone and piericidin A [Murai, M., et al. (2007) Biochemistry 46 , 6409-6416]. As further exploration of
Protocols
Enzymatic Assay of Superoxide Dismutase
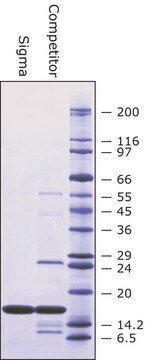
Separation of Superoxide dismutase
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service