S5395
Superoxide Dismutase from bovine erythrocytes
≥3,000 units/mg protein, BioReagent, lyophilized powder, suitable for cell culture
Synonym(s):
SOD, Superoxide: superoxide oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
biological source
bovine erythrocytes
Quality Level
product line
BioReagent
form
lyophilized powder
specific activity
≥3,000 units/mg protein
mol wt
32.5 kDa
packaging
pkg of 15000 units
technique(s)
cell culture | mammalian: suitable
pH
7.6-10.5
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
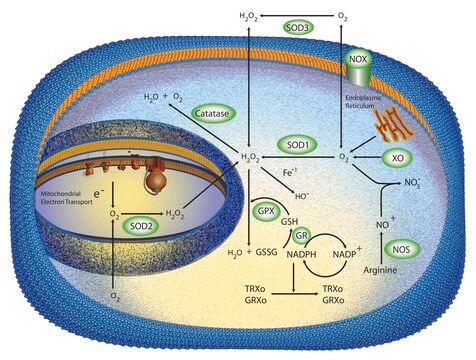
Superoxide Dismutase from bovine erythrocytes is a metalloprotein which disproportionates superoxide anion radicals. It is a 31.5 kDa copper binding protein and displays a conserved domain and fold. It is a homodimer with one copper and zinc ion per subunit and has antiparallel “greek-key” β barrel fold.
Application
Superoxide Dismutase (SOD) from bovine erythrocytes has been used:
- for measuring the superoxide radical using the electron paramagnetic resonance spin in human brain microvascular endothelial cells
- for measuring superoxide production in cytochrome C assay in peripheral blood mononuclear cells
- as a standard in characterization of hen egg SOD using Fourier-transform infrared spectroscopy (FTIR) and matrix-assisted laser desorption/ionization (MALDI) analysis
Biochem/physiol Actions
Superoxide Dismutase from bovine erythrocytes catalyzes the dismutation of superoxide radicals to hydrogen peroxide and molecular oxygen. It serves as an antioxidant and plays a critical role in the defense of cells against the toxic effects of oxygen radicals. Competes with nitric oxide (NO) for superoxide anion (which reacts with NO to form peroxynitrite), thereby SOD promotes the activity of NO. SOD has also been shown to suppress apoptosis in cultured rat ovarian follicles, neural cell lines, and transgenic mice.
Unit Definition
One unit will inhibit reduction of cytochrome c by 50% in a coupled system with xanthine oxidase at pH 7.8 at 25 °C in a 3.0 mL reaction volume. Xanthine oxidase concentration should produce an initial ΔA550 of 0.025 ± 0.005 per min.
Analysis Note
For assay method, see McCord, J.M. and Fridovich,I., J. Biol. Chem., 244, 6049 (1969).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chunxia Xiao et al.
PloS one, 7(1), e30343-e30343 (2012-01-25)
The C57BLKS/J db/db (db/db) mouse is a widely used type 2 diabetic animal model, and this model develops early inner retinal neuronal dysfunction beginning at 24 weeks. The neural mechanisms that mediate early stage retinal dysfunction in this model are
Zoe Loomis et al.
PloS one, 12(2), e0171219-e0171219 (2017-02-06)
It is now well established that both inherited and acquired forms of hemolytic disease can promote pulmonary vascular disease consequent of free hemoglobin (Hb) induced NO scavenging, elevations in reactive oxygen species and lipid peroxidation. It has recently been reported
Partial biochemical characterization of Cu, Zn-superoxide dismutase extracted from eggs of hens (Gallus gallus domesticus)
Wawrzykowski J and Kankofer M
Food Chemistry, 227, 390-396 (2017)
human cystic fibrosis macrophages have defective calcium-dependent protein kinase C activation of the NADPH oxidase, an effect augmented by Burkholderia cenocepacia
Assani K, et al.
Journal of immunology (Baltimore, Md. : 1950), 198(5), 1985-1994 (2017)
Structure of fully reduced bovine copper zinc superoxide dismutase at 1.15
Hough MA and Hasnain SS
Structure, 11(8), 937-946 (2003)
Protocols
Enzymatic Assay of Superoxide Dismutase
Separation of Superoxide dismutase
Chromatograms
application for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service