Safety is Everything
Biologics testing partner of choice
BioReliance® Contract Testing Services
Choosing the right partner for analytical and biosafety testing is critical in the race to approval. Our BioReliance® Contract Testing Services offer exceptional, risk-mitigating solutions with technical and regulatory expertise, to help bring life-changing drugs to market.
Our testing and manufacturing services span the product cycle from early pre-clinical development to licensed production. Clients partner with us to meet their needs for biologics safety testing, analytical development, and biomanufacturing services.
A Reputation for Shaping the Biotesting Industry
We’re entrusted with the safety of the world's biologics because for us, safety is everything. We balance risk to optimize speed to market without ever compromising on safety or quality. Our strength is our people, and with 75+ years of trusted expertise, we’re the partner of choice for biomanufacturers.
Our proudest innovations include:
1955
Developed polio vaccine biosafety protocols
1960
Developed mouse antibody test in collaboration with the U.S. NIH
1983
Started biosafety testing for the first licensed monoclonal antibody product
2007
Characterized the first U.S. national stem cell banks
2023
Leveraging the power of Next Generation Sequencing (NGS) and our proprietary Blazar® platform to accelerate testing and reduce the use of animals
The Value We Provide
Known for our broad portfolio & expertise – with standard setting innovation & template development to push the industry forward.
Flexibility through manufacturing and testing capacity – making significant investment in our capabilities and capacity to scale with client's complex supply chain needs.
Decades of experience to inform and navigate client’s pathways to approval. Our global regulatory know-how ensures quality standards and compliance are met.
Across the biosafety and regulatory landscape

Analytical Development
Orthogonal approaches to product characterization support better understanding of an asset's performance, safety, and the manufacturing processes for Drug Substance (DS) and Drug Product (DP).
Identity, purity, strength, and potency should be considered collectively to inform the best decisions about: Where and how to act, setting specifications, and understanding performance.
A subset of approaches should move into Analytical Development to formalize assays to support testing under GMP required for Critical Quality Attributes (CQAs) defined by ICH Q6B.
It is important to recognize that all modalities and processes are different, but the attributes defining quality are consistent and must be understood and monitored.
ADCs
With ADCs, it is important to look beyond drug-to-antibody ratio (DAR). Understanding how post- translational modifications and conjugation may affect binding and potency is an important consideration that affects internalization and ultimately, potency.
mAbs
How well do you understand the structure-function relationship with your molecule? With mAbs, it is important to understand binding performance, covalent modifications (post translational modifications and/or unintentional modifications) and how those factors affect performance.
mRNA
Given the unique characteristics of the mRNA/LNP complex, new expertise and a series of novel analytical capabilities are required to ensure fast, efficacious, and compliant mRNA-based vaccines and therapeutics. We provide testing services for mRNA starting materials, drug substance, and drug product.
Cell & Gene Therapy
AAV therapies are complex and present unique challenges to monitor quality and performance. These activities require careful planning and management of orthogonal techniques (e.g., analytical, structural, and molecular). Breadth of capabilities and coordination are key to success.

Raw Material Testing
Use of animal-derived products in biologics development or manufacturing creates an inherent risk of adventitious agent contamination in historic cell banks, seed viruses, and finished products. Our wide range of testing services are aimed at ensuring the quality and purity of raw materials.

Cell and Viral Bank Testing
Cell and virus bank is the starting material for the biologics manufacturing process. Extensive characterization at this stage is important to ensure viral safety and genetic stability.
We leverage decades of experience in testing at every phase from pre-clinical through commercialization to deliver comprehensive testing and expert regulatory guidance.
We partner with clients to perform a risk assessment to determine the factors that may influence the potential level of infectious particles to effectively characterize the cell line.

Viral Clearance Testing
Our viral clearance studies are designed by experts in global regulatory requirements, downstream processing, and virology. Having performed more than 17,300 viral clearance studies in our U.S., UK, China, and Singapore facilities, biomanufacturers trust in us to minimize risk as they bring their products to market.
Provise™ Clearance Service
Our Provise™ Clearance Service deploys a team of highly trained & experienced process scientists and study directors on our clients’ behalf to perform all required process steps at our state-of-the-art facilities. We provide updates at every step without the need for clients to leave their workplace, maximizing their team’s productivity. Our clients receive a QA-audited final report to expertly support their regulatory filing.
Standard & Hybrid Clearance Services
Booking time at one of our facilities to execute a viral clearance study offers an optimal combination of flexibility, productivity, and quality assurance. In a user-friendly setting featuring the latest technology, transparency, and choice of standard or hybrid clearance packages, our team provides precisely the level of support required when in pursuit of IND or BLA filing.

Rapid Molecular Detection
We have a history of shaping the biosafety testing industry with new technologies and paradigms. Our scientific, regulatory, and quality assurance experts have developed and guide in the selection of rapid detection methods to accelerate biosafety testing packages, while addressing issues with limited sample volume.
The Blazar® Platform - Rapid Molecular Detection
Our proprietary Blazar® platform provides accurate and highly sensitive viral detection in just days. Combining the breadth of detection of next generation sequencing (NGS) with the speed and sensitivity of PCR methods, the Blazar® platform relies on a degenerate PCR approach for coverage of more than 5,000 viral variants. By amplifying multiple targets within a conserved region of the viral family genome, our clients can detect a much broader range of adventitious viruses than with traditional PCR methods.
Next Generation Sequencing
Revolutionizing the development and analysis of biologic therapeutics, next generation sequencing (NGS) allows our clients to meet the challenge of rapidly characterizing their products, while identifying known and unknown agents with pinpoint precision and accuracy. Our suite of NGS services is based on complementary technology platforms that rapidly generate deep sequencing datasets for comprehensive data analysis, backed by biosafety experts who deliver intelligent results tailored to our clients’ unique testing needs.

Bulk Harvest Release Testing
Whether preparing for clinic or manufacturing a life-saving therapeutic biologic, it is critical that our clients protect their investment with GMP-compliant release testing.
We provide GMP-compliant testing for unprocessed and purified bulk harvest to meet the requirements of their biologic for preclinical and clinical studies, as well as licensed biologics.
mAbs
Cells, spent media, the biologic, and any by-products of the reaction, require rigorous testing to confirm the absence of any adventitious agents and ensure its suitability for downstream processing. Failure to identify contamination or investigate unexpected results at this early stage of the biomanufacturing process can lead to catastrophic downstream failure.
Cell and Gene Therapy
To unlock the potential of gene therapies and gene-modified cell therapies, our testing services often play a critical role in helping biomanufacturers ensure the identity, potency, and/or safety of the unpurified bulk, raw materials, cell banks, virus banks, and plasmids that are used in production of their final products. We offer cGMP-compliant support for cell and gene therapies, including those that utilize AAV, Retrovirus/Lentivirus, and Adenovirus.

Drug Product Release Testing
To help our clients demonstrate compliance with regulatory guidelines prior to releasing their biologics into global pharmaceutical markets, we offer a wide range of GMP assays and testing services for final product packages.
mAbs
Every lot of monoclonal antibodies produced for pre-clinical and clinical studies requires a series of tests to ensure that active ingredients are free of contaminants according to 21 CFR 211.165 and 21 CFR 610. Our comprehensive spectrum of GMP-compliant final product testing services provides assurance of patient safety and regulatory compliance in any geography, with service provision on three continents and backup facilities to ensure continuity of supply.
Cell and Gene Therapy
Our team of experts consult with our clients to develop a specific testing program, drawing upon an array of available platform assays for final product release. We can help navigate the unique logistic, regulatory, and technical requirements of the advanced therapy with biosafety and characterization testing methods.
Support for all phases of biopharmaceutical drug development
In any stage of the biopharma development process, our leading GMP-compliant biosafety services and regulatory knowledge can help biomanufacturers progress their therapeutic from discovery to release.
*Microbiology assays and other methods for adventitious agent detection
**Support for IND and BLA enabling studies
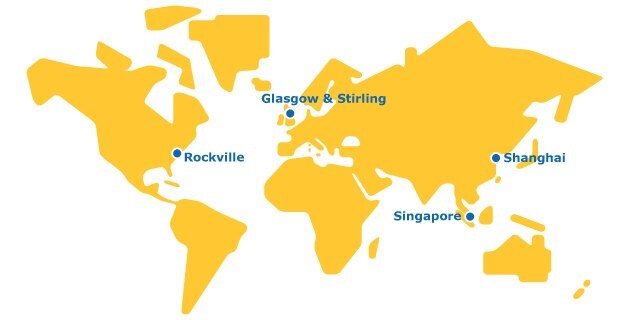
A Global Footprint
We are a single organization with a global network to deliver testing services across all stages of the molecule value chain.
Our state-of-the-art testing network consists of four major sites near global biopharma hubs. Ongoing investments to expand capacity across all locations will help us meet the growing demand for robust biosafety testing studies for traditional and novel therapies.

Our Shanghai site is home to our China Biologics Testing Center, which houses viral clearance suites. This enables clients to locally conduct viral clearance studies from pre-clinical development to commercialization for in China and for global export.

Our biosafety testing service site houses our cell bank manufacturing, biorepository, cell line characterization, and drug substance and drug product release testing services.

Our site features viral clearance study suites. Our Provise™ Clearance Service deploys a team of highly trained & experienced process scientists and study directors on your behalf, to perform all your required process steps at our state-of-the-art Stirling facility.

Our Biosafety testing services site conducts cell line characterization, viral clearance, and drug substance and drug product release testing.

Biosafety testing and manufacturing services span the product cycle from early pre-clinical development to licensed production. Services include analytical development, cell banking, cell line characterization, viral clearance, drug substance and drug product release testing, as well as rapid methods.
To continue reading please sign in or create an account.
Don't Have An Account?