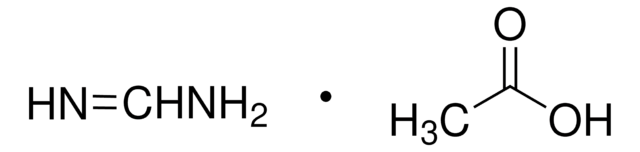All Photos(1)
About This Item
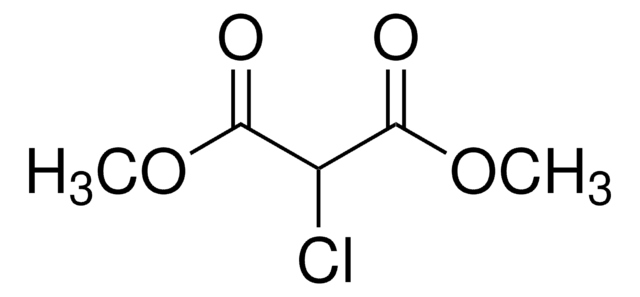
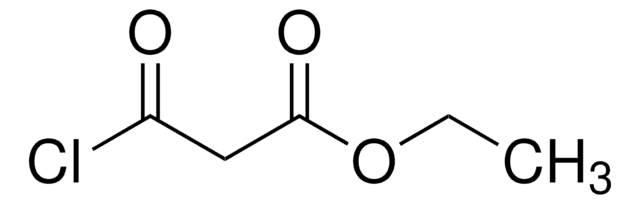
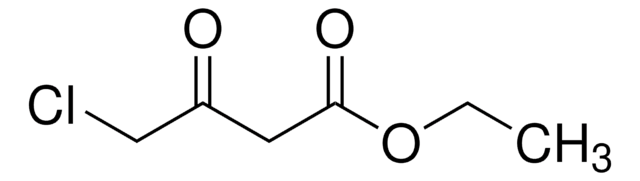
Linear Formula:
ClCH(CO2C2H5)2
CAS Number:
Molecular Weight:
194.61
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.432 (lit.)
density
1.204 g/mL at 25 °C (lit.)
functional group
chloro
ester
SMILES string
CCOC(=O)C(Cl)C(=O)OCC
InChI
1S/C7H11ClO4/c1-3-11-6(9)5(8)7(10)12-4-2/h5H,3-4H2,1-2H3
InChI key
WLWCQKMQYZFTDR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Diethyl chloromalonate (Diethyl α-chloromalonate) is a 2-halo-1,3-dicarbonyl compound. It participates in K2CO3-catalyzed domino reactions (Michael alkylation, Mannich alkylation, and aldol alkylation) of salicylic aldehyde derivatives to afford functionalized 2,3-dihydrobenzofurans. It reacts with Cs2CO3 in the presence of elemental S8 or Sen to afford the corresponding diethyl thioxo- or selenoxomalonates, which can be trapped in situ with various 1,3-dienes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R N Henrie et al.
Journal of medicinal chemistry, 26(4), 559-563 (1983-04-01)
Reaction of diethyl chloromalonate with beta-mercapto amines, 9, gave 1,4-thiazin-3-ones, 10, which were alkylated exclusively at the lactam oxygen with triethyloxonium tetrafluoroborate and subsequently condensed with guanidine to give the first reported 5-thiapterins, 8. Oxidation of 8 with m-chloroperoxybenzoic acid
Preparation of cycloaddition chemistry of thio-and selenocarbonyls derived from reaction of elemental sulfur and selenium with stabilized a-halo anions.
Abelman MM.
Tetrahedron Letters, 32(50), 7389-7392 (1990)
Qu-Bo Li et al.
The Journal of organic chemistry, 76(17), 7222-7228 (2011-07-29)
The K(2)CO(3)-catalyzed domino reactions (Michael alkylation, Mannich alkylation, and aldol alkylation) of salicylic aldehyde derivatives (2-hydroxyaryl-α,β-unsaturated ketones, 2-hydroxyarylnitroalkenes, 2-hydroxyarylimines, and salicylic aldehydes) and 2-halo-1,3-dicarbonyl compounds (diethyl α-bromomalonate, diethyl α-chloromalonate, ethyl 2-chloroacetoacetate, and 3-chloropentane-2,4-dione) were carried out under mild conditions to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service