F15803
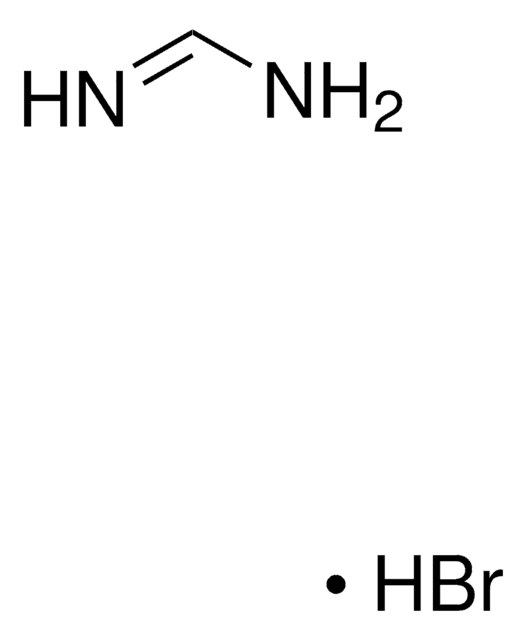
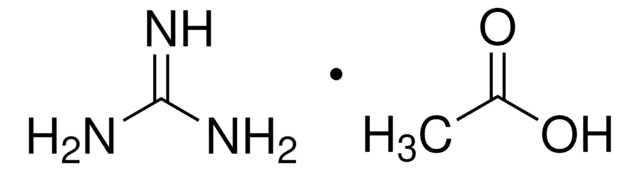
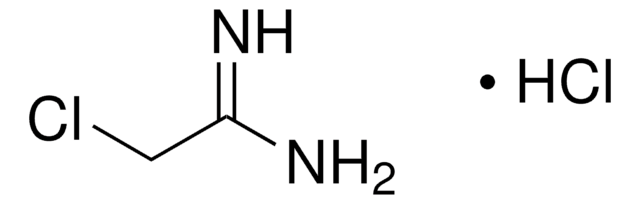
Formamidine acetate salt
99%
Synonym(s):
Formamidine acetic acid salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
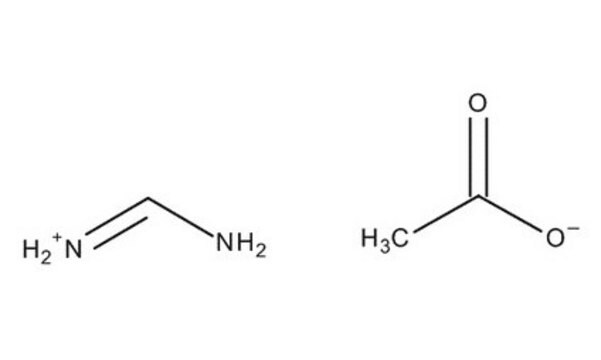
HN=CHNH2 · CH3COOH
CAS Number:
Molecular Weight:
104.11
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
158-161 °C (dec.) (lit.)
SMILES string
[H]C(N)=N.CC(O)=O
InChI
1S/C2H4O2.CH4N2/c1-2(3)4;2-1-3/h1H3,(H,3,4);1H,(H3,2,3)
InChI key
XPOLVIIHTDKJRY-UHFFFAOYSA-N
General description
Formamidine acetate salt is an organic compound commonly used as a reagent in the synthesis of heterocyclic compounds.
Application
Formamidine acetate salt is widely used in the preparation of formamidinium lead triiodide (FAPbI3) perovskites for perovskite solar cells. It is also used in the synthesis of formamidinium lead bromide nanocrystals for use as emitters in display applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Monodisperse formamidinium lead bromide nanocrystals with bright and stable green photoluminescence
Protesescu L, et al.
Journal of the American Chemical Society, 138(43), 14202-14205 (2016)
Nanowire lasers of formamidinium lead halide perovskites and their stabilized alloys with improved stability
Fu Y, et al.
Nano Letters, 16(2), 1000-1008 (2016)
Additive-modulated evolution of HC (NH2) 2PbI3 black polymorph for mesoscopic perovskite solar cells
Wang Z, et al.
Chemistry of Materials, 27(20), 7149-7155 (2015)
He Huang et al.
Nanomaterials (Basel, Switzerland), 10(1) (2020-01-08)
Metal halide perovskites are promising materials for a range of applications. The synthesis of light-emitting perovskite nanorods has become popular recently. Thus far, the facile synthesis of perovskite nanorods remains elusive. In this work, we have developed a facile synthesis
Xiaoqing Jiang et al.
Scientific reports, 7, 42564-42564 (2017-02-18)
Herein, we successfully applied a facile in-situ solid-state synthesis of conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) as a HTM, directly on top of the perovskite layer, in conventional mesoscopic perovskite solar cells (PSCs) (n-i-p structure). The fabrication of the PEDOT film only
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service