160695
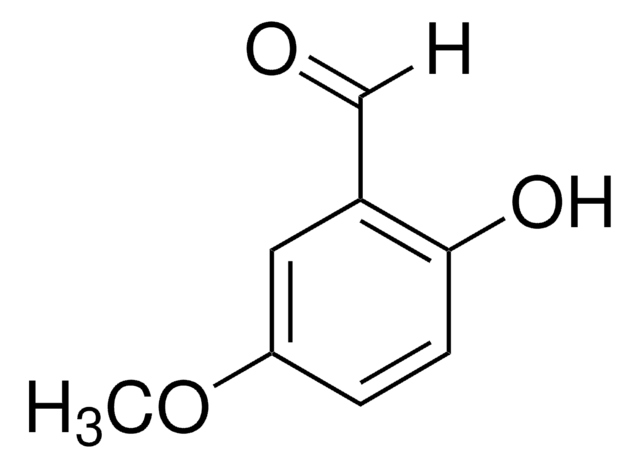
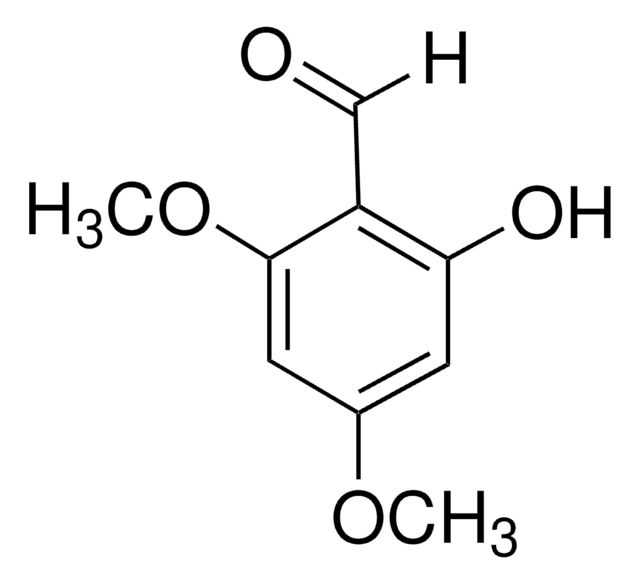
2-Hydroxy-4-methoxybenzaldehyde
98%
Synonym(s):
4-Methoxysalicylaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
152.15
Beilstein:
1072443
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
41-43 °C (lit.)
SMILES string
[H]C(=O)c1ccc(OC)cc1O
InChI
1S/C8H8O3/c1-11-7-3-2-6(5-9)8(10)4-7/h2-5,10H,1H3
InChI key
WZUODJNEIXSNEU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Hydroxy-4-methoxybenzaldehyde is the main component of root bark essential oil of Periploca sepium Bunge. It is a potential tyrosinase inhibitor present in African medicinal plants.
Application
2-Hydroxy-4-methoxybenzaldehyde was used in the synthesis of Schiff base ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gholamreza Karimipour et al.
Biological trace element research, 145(1), 109-117 (2011-08-13)
In this study, a new sorbent based on the gold nanoparticle loaded in activated carbon (Au-NP-AC) was synthesized and modified by bis(4-methoxy salicylaldehyde)-1,2-phenylenediamine (BMSAPD). This sorbent, which is abbreviated as Au-NP-AC-BMSAPD, has been applied for the enrichment and preconcentration of
Ken-ichi Nihei et al.
Bioorganic & medicinal chemistry letters, 14(3), 681-683 (2004-01-27)
Chamaecin (2-hydroxy-4-isopropylbenzaldehyde) was synthesized and tested for its tyrosinase inhibitory activity. It partially inhibits the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) catalyzed by mushroom tyrosinase with an IC(50) of 2.3 microM. The inhibition kinetics analyzed by Dixon plots found that chamaecin is
Rubén Córdoba et al.
Bioorganic & medicinal chemistry, 15(15), 5300-5315 (2007-05-18)
A series of analogues of the potentially angiogenic inhibitor aeroplysinin-1 1 were synthesized and their in vitro antiangiogenic and cytotoxic activities evaluated. In the case of epoxy ketone 6 and azlactone 36 the relationship sprouting inhibition assay/cytotoxicity in BAE cells
Jihua Wang et al.
Molecules (Basel, Switzerland), 15(8), 5807-5817 (2010-08-26)
The root bark essential oil of Periploca sepium Bunge (Asclepiadaceae/ Apocynaceae) obtained by hydrodistillation was investigated by GC and GC-MS. 2-Hydroxy-4-methoxybenzaldehyde was found to be the main component (78.8% of the total) among 17 identified compounds. 2-Hydroxy-4-methoxybenzaldehyde was separated and
Synthesis, crystal structures and fluorescence properties of two new di-and polynuclear Cd (II) complexes with N2O donor set of a tridentate Schiff base ligand.
Basak S, et al.
Polyhedron, 27(4), 1193-1200 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service