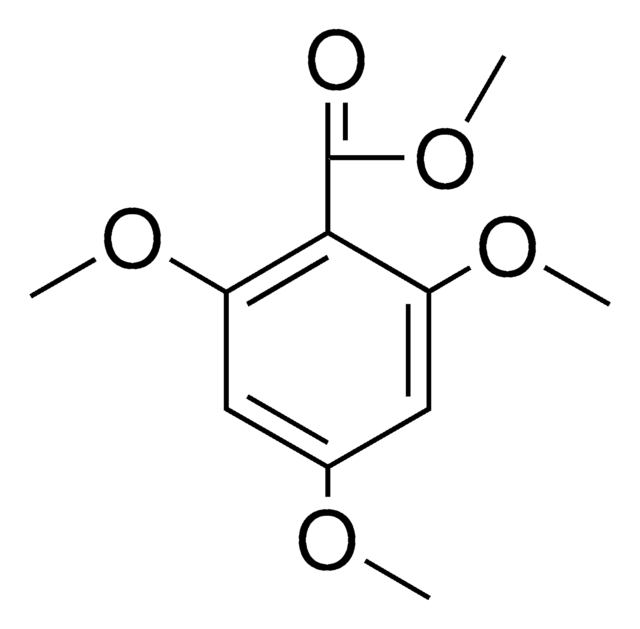
138797
4,6-Dimethoxysalicylaldehyde
98%
Synonym(s):
2,4-Dimethoxy-6-hydroxybenzaldehyde, 4,6-Dimethoxy-2-hydroxybenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
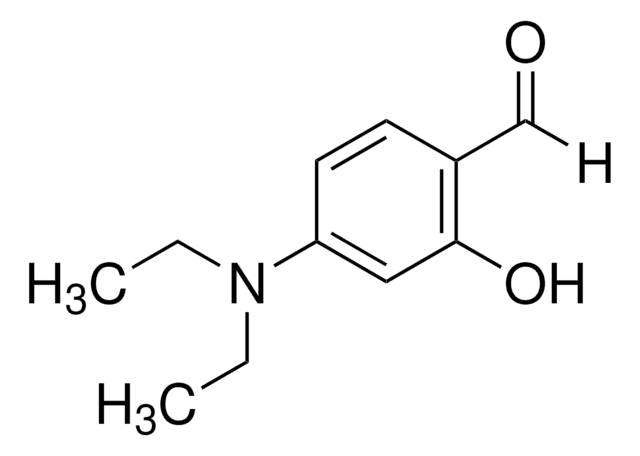
HOC6H2(OCH3)2CHO
CAS Number:
Molecular Weight:
182.17
Beilstein:
1241679
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
68-70 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1c(O)cc(OC)cc1OC
InChI
1S/C9H10O4/c1-12-6-3-8(11)7(5-10)9(4-6)13-2/h3-5,11H,1-2H3
InChI key
FQRQWPNYJOFDLO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4,6-Dimethoxysalicylaldehyde on condensation with methylamine yields Schiff bases.
Application
4,6-Dimethoxysalicylaldehyde was used in the preparation of a new class of efficient ketocoumarin triplet sensitizers. It was used as staring reagent in the total synthesis of (+/-)-linderol A, a hexahydrodibenzofuran.
Biochem/physiol Actions
4,6-Dimethoxysalicylaldehyde has antimicrobial activity against Candida albicans.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wojciech Schilf et al.
Magnetic resonance in chemistry : MRC, 42(6), 556-560 (2004-05-12)
Hydrogen bonding within the structures of three Schiff bases (1-3), obtained by condensation of 4-methoxy-, 5-methoxy- and 4,6-dimethoxysalicylaldehyde with methylamine, was investigated by measuring deuterium and tritium NMR isotope effects. The primary deuterium and tritium isotope effects (delta(XH)-delta(XD/T)) and secondary
Eila Pelttari et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 62(7-8), 487-497 (2007-10-05)
A systematic survey of the antimicrobial properties of substituted salicylaldehydes and some related aromatic aldehydes is reported. A total of 23 different compounds, each at four different concentrations, were studied using a panel of seven microbes (Aspergillus niger, Bacillus cereus
Ketocoumarins: a new class of triplet sensitizers.
Specht DP, et al.
Tetrahedron, 38(9), 1203-1211 (1982)
M Yamashita et al.
Organic letters, 3(9), 1359-1362 (2001-05-12)
[reaction in text] The first total synthesis of (+/-)-linderol A, a hexahydrodibenzofuran isolated from Lindera umbellata bark, with potent inhibitory activity on melanin biosynthesis of cultured B-16 melanoma cells was achieved via a 20-step of reaction in 7.64% overall yield
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 138797-1G | 4061825582979 |
| 138797-250MG |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![2,3,6,7-Tetrahydro-8-hydroxy-1H,5H-benzo[ij]quinolizine-9-carboxaldehyde 98%](/deepweb/assets/sigmaaldrich/product/structures/166/830/a0d9a84a-5623-41a1-a54b-3b0272e5b28c/640/a0d9a84a-5623-41a1-a54b-3b0272e5b28c.png)
![2-[(Dimethylamino)methyl]phenol AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/367/175/0ca3106b-78d7-4d06-ab7f-d960099c947d/640/0ca3106b-78d7-4d06-ab7f-d960099c947d.png)