N9150
Nystatin
ready made solution, suitable for cell culture
Sinónimos:
Nystatin, Fungicidin, Mycostatin
About This Item
Productos recomendados
product name
Nystatin Ready made solution, suitable for cell culture
biological source
Streptomyces noursei
form
solution
technique(s)
cell culture | mammalian: suitable
shipped in
dry ice
storage temp.
−20°C
SMILES string
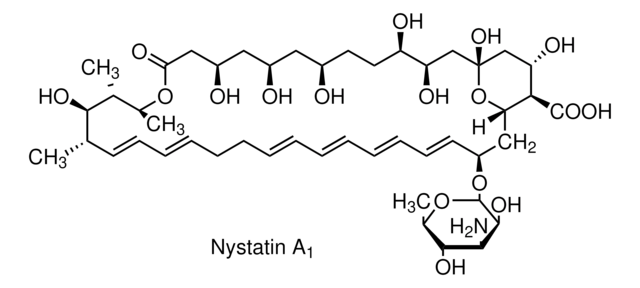
O[C@@H]([C@H](C)[C@H](C)O1)[C@@H](C)/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@]3([H])[C@H](C(O)=O)[C@@H](O)C[C@](O)(O3)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)C[C@@H](O)CC1=O
InChI
1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28+,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1
InChI key
VQOXZBDYSJBXMA-QEKUPDCNSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Biochem/physiol Actions
Antimicrobial spectrum: Nystatin acts against fungi, yeasts and molds.
Features and Benefits
- Nystatin ready made solution is a fully solubilized formulation of Nystatin with potency of 8000-13000 units/ml according to USP standard.
- Nystatin ready made solution is 0.2μm filtered, endotoxin.
- The suggested working concentration of Nystatin ready made solution is 30 units/ml.
- The ready made solution is light sensitive. It is recommended to store the solution in the dark.
- It is recommended to avoid freeze thaw cycles of this product. Therefore, it is recommended to aliquot and store at -20°C.
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico