N6261
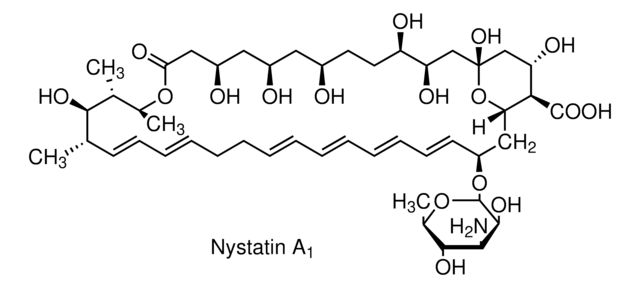
Nystatin
powder, suitable for cell culture, BioReagent
Sinónimos:
Fungicidin, Mycostatin
About This Item
Productos recomendados
product name
Nystatin, powder, BioReagent, suitable for cell culture
product line
BioReagent
Quality Level
form
powder
potency
≥4,400 per mg
technique(s)
cell culture | mammalian: suitable
solubility
H2O: insoluble
antibiotic activity spectrum
fungi
yeast
mode of action
cell membrane | interferes
storage temp.
−20°C
SMILES string
O[C@@H]([C@H](C)[C@H](C)O1)[C@@H](C)/C=C/C=C/CC/C=C/C=C/C=C/C=C/[C@H](O[C@@H]2O[C@H](C)[C@@H](O)[C@H](N)[C@@H]2O)C[C@@]3([H])[C@H](C(O)=O)[C@@H](O)C[C@](O)(O3)C[C@@H](O)[C@H](O)CC[C@@H](O)C[C@@H](O)C[C@@H](O)CC1=O
InChI
1S/C47H75NO17/c1-27-17-15-13-11-9-7-5-6-8-10-12-14-16-18-34(64-46-44(58)41(48)43(57)30(4)63-46)24-38-40(45(59)60)37(54)26-47(61,65-38)25-36(53)35(52)20-19-31(49)21-32(50)22-33(51)23-39(55)62-29(3)28(2)42(27)56/h5-6,8,10-18,27-38,40-44,46,49-54,56-58,61H,7,9,19-26,48H2,1-4H3,(H,59,60)/b6-5+,10-8+,13-11+,14-12+,17-15+,18-16+/t27-,28+,29-,30+,31+,32+,33+,34-,35+,36+,37-,38-,40+,41-,42+,43+,44-,46-,47+/m0/s1
InChI key
VQOXZBDYSJBXMA-QEKUPDCNSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- for the treatment of human macrophage cell line U937, to study the effect of nystatin on the production of IL-8 (interleukin 8) by silver nanoparticle-treated U937 cell line
- to determine its effect on intracellular accumulation of OxLDL (oxidized low density lipoprotein) mediated by SR-BI (scavenger receptor class B type I) in COS-7 cell line
- as a lipid raft modulator on mice macrophages to study the relationship between increased number of lipid rafts and the heightened response of TNF-α (tumor necrosis factor)
Biochem/physiol Actions
Antimicrobial spectrum: Nystatin acts against fungi, yeasts and molds.
Caution
Preparation Note
Other Notes
Related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico