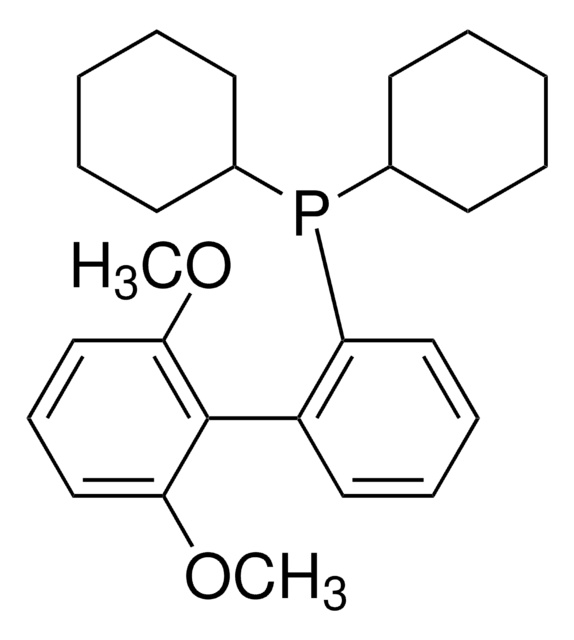
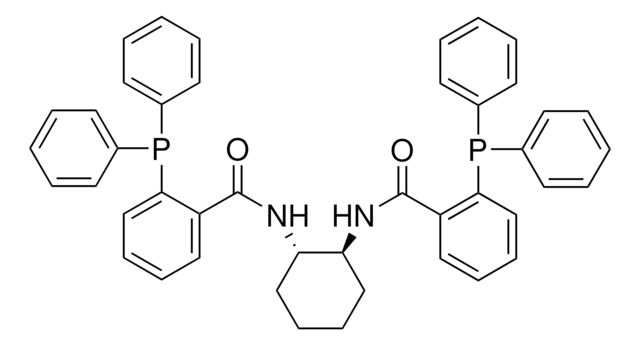
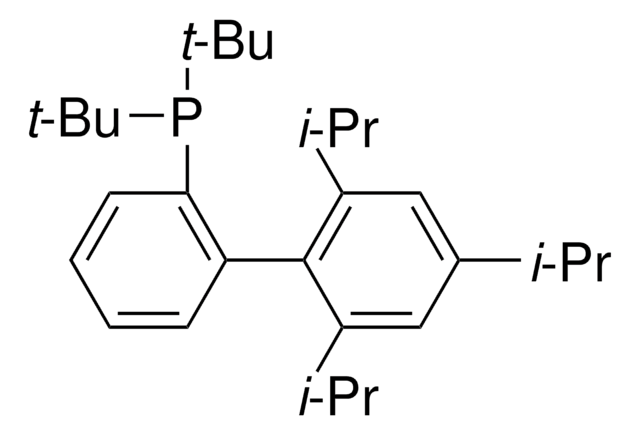
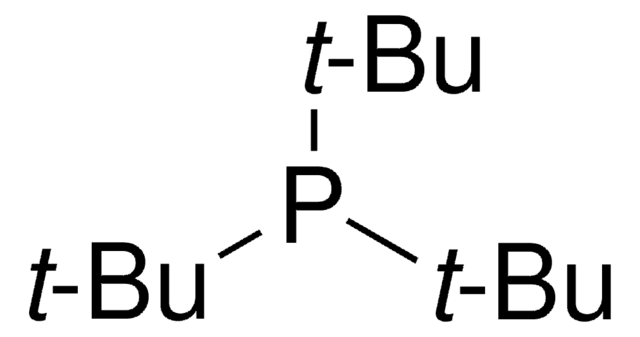366315
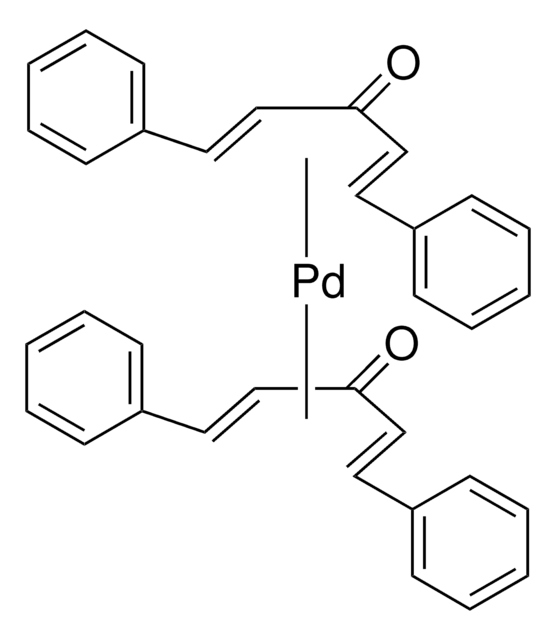
Tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct
Sinónimos:
Dipalladium-tris(dibenzylideneacetone)chloroform complex, Pd2(dba)3 · CHCl3
About This Item
Productos recomendados
form
solid
Quality Level
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
131-135 °C (lit.)
SMILES string
[Pd].[Pd].ClC(Cl)Cl.O=C(/C=C/c1ccccc1)\C=C\c2ccccc2.O=C(/C=C/c3ccccc3)\C=C\c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.CHCl3.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;2-1(3)4;;/h3*1-14H;1H;;/b3*13-11+,14-12+;;;
InChI key
LNAMMBFJMYMQTO-FNEBRGMMSA-N
General description
Application
- To compose the catalytic system for the preparation of homoallylpalladium complexes.These complexes underwent in situ Stille type cross coupling with various vinyltin reagents to afford the cyclized products bearing allyl appendages.
- As palladium source in the asymmetric transformations of 3,4-epoxy-1-butene.
- As catalyst for the Heck cross-coupling reaction of iodobenzene with styrene.
- As cyclization catalyst.
- As catalyst for [2+2+2] cycloaddtion of didehydrotriphenylenes to the corresponding extended triphenylenes.
- As catalyst for the carbonylation of b,b-imidoyl iodides to the corresponding imidate esters used, in turn, to prepare cyclic, quaternary amino acids.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Carc. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)