208671
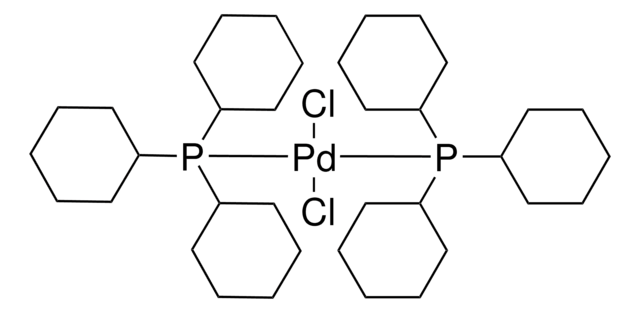
Bis(triphenylphosphine)palladium(II) dichloride
98%
Sinónimos:
Dichlorobis(triphenylphosphine)palladium(II), Palladium(II)bis(triphenylphosphine) dichloride, PdCl2(PPh3)2
About This Item
Productos recomendados
Quality Level
assay
98%
form
solid
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
SMILES string
Cl[Pd]Cl.c1(P(c2ccccc2)c3ccccc3)ccccc1.c4(P(c5ccccc5)c6ccccc6)ccccc4
InChI
1S/2C18H15P.2ClH.Pd/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2
InChI key
YNHIGQDRGKUECZ-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Coupling of 2-iodoanisole and terminal alkynes to synthesize 2,3-disubstituted benzofurans.
- Copper-free Sonogashira cross-coupling reaction to synthesize diphenylacetylene.
- Regioselective hydrocarboxylation of styrene.
- Negishi coupling of fluoroarylzinc pivalates to prepare fluorinated oligophenyls.
- Coupling of iodo-α-β-unsaturated esters to afford tetrasubstituted olefins.
Related product
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 4 - Skin Sens. 1A
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)


![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)