914924
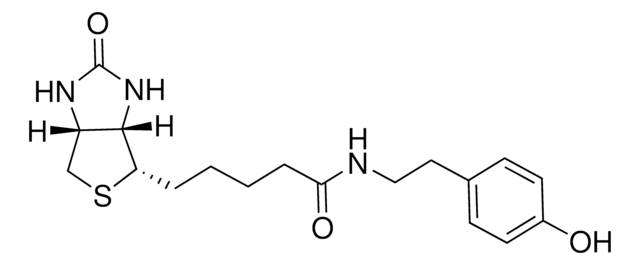
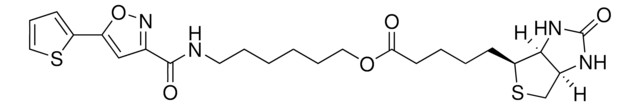
3-[2-N-(Biotinyl)aminoethyldithio]propanoic acid
≥95%
Synonym(s):
3-((2-(5-((3aS,4S,6aR)-2-Oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)ethyl)disulfaneyl)propanoic acid, Biotin-SS-COOH, Cleavable biotin linker
About This Item
Recommended Products
Quality Level
Assay
≥95%
form
powder
mp
172-175 °C
storage temp.
−20°C
SMILES string
S1[C@H]([C@H]2NC(=O)N[C@H]2C1)CCCCC(=O)NCCSSCCC(=O)O
InChI
1S/C15H25N3O4S3/c19-12(16-6-8-25-24-7-5-13(20)21)4-2-1-3-11-14-10(9-23-11)17-15(22)18-14/h10-11,14H,1-9H2,(H,16,19)(H,20,21)(H2,17,18,22)/t10-,11-,14-/m0/s1
InChI key
LUKYYZVIDAWYMZ-MJVIPROJSA-N
Application
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
A Mechanism-Based ICAT Strategy for Comparing Relative Expression and Activity Levels of Glycosidases in Biological Systems
Dissociation-independent selection of high-affinity anti-hapten phage antibodies using cleavable biotin-conjugated haptens
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![Spiro[9H-fluorene-9,9′-[9H]xanthene]-2,7-diamine](/deepweb/assets/sigmaaldrich/product/structures/307/234/46c07f0c-9242-4c0b-8994-8121690da3c9/640/46c07f0c-9242-4c0b-8994-8121690da3c9.png)