1134153
USP
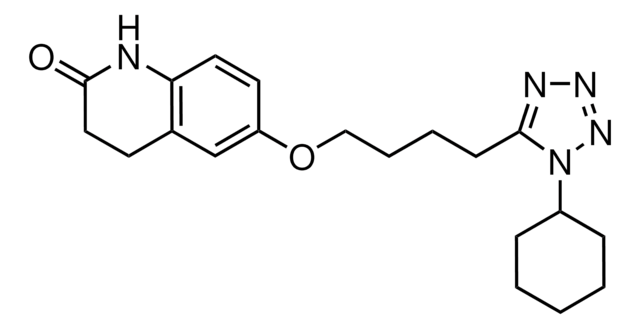
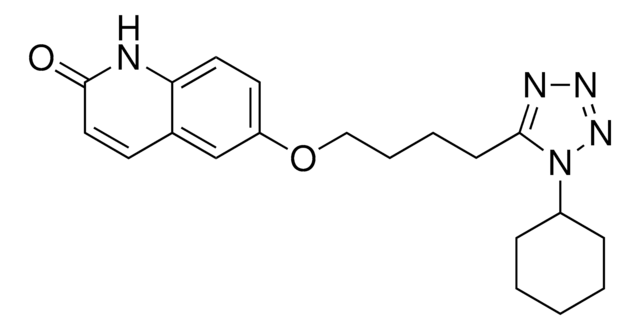
Cilostazol
United States Pharmacopeia (USP) Reference Standard
동의어(들):
6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-3,4-dihydro-2(1H)-quinolinone, OPC 13013, OPC 21, Pletaal
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C20H27N5O2
CAS Number:
Molecular Weight:
369.46
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
cilostazol
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
O=C1CCc2cc(OCCCCc3nnnn3C4CCCCC4)ccc2N1
InChI
1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
InChI key
RRGUKTPIGVIEKM-UHFFFAOYSA-N
유전자 정보
human ... PDE3A(5139)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Cilostazol is a potent cyclic nucleotide phosphodiesterase inhibitor. It is mainly used as antiplatelet agent.
애플리케이션
Cilostazol USP Reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monograph such as Cilostazol Tablets
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
S Takahashi et al.
Journal of cardiovascular pharmacology, 20(6), 900-906 (1992-12-01)
Cilostazol, a cyclic AMP phosphodiesterase inhibitor, has been used as an antiplatelet agent. In the present study, we investigated the in vitro effect of cilostazol on DNA synthesis in rat aortic arterial smooth muscle cells (SMCs) in culture stimulated with
Eun-Seok Shin et al.
Heart (British Cardiac Society), 100(19), 1531-1536 (2014-06-18)
We conducted a randomised, double blind, placebo controlled trial to assess the efficacy and safety of cilostazol, a selective inhibitor of phosphodiesterase 3, in patients with vasospastic angina (VSA). Cilostazol has been shown to induce vascular dilatation, but its efficacy
Haeyeon Hong et al.
Clinical therapeutics, 36(8), 1290-1301 (2014-07-12)
Despite numerous efforts to develop effective medications for the treatment of intermittent claudication (IC) over the past 4 decades, a gold standard medical management option has yet to be defined. Although not life-threatening, IC interferes with mobility and activities of
Alexander J Ansara et al.
The Annals of pharmacotherapy, 46(3), 394-402 (2012-02-23)
To evaluate the safety and efficacy of cilostazol for secondary prevention of non-cardioembolic ischemic stroke. PubMed and MEDLINE searches were performed (January 1970-September 2011) using the key words cilostazol, antiplatelet, aspirin, acetylsalicylic acid, secondary stroke prevention, ischemic stroke, intracerebral hemorrhage
Natnicha Kanlop et al.
Journal of cardiovascular medicine (Hagerstown, Md.), 12(2), 88-95 (2011-01-05)
Cilostazol is a selective phosphodiesterase 3 (PDE3) inhibitor approved by the Food and Drug Administration for treatment of intermittent claudication. It has also been used in bradyarrhythmic patients to increase heart rates. Recently, cilostazol has been shown to prevent ventricular
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.